Groundbreaking Biologic Dupixent Shows Promising Results in Bullous Pemphigoid Study
A crucial clinical trial (ADEPT) for Dupixent (dupilumab) in the treatment of bullous pemphigoid (BP) reached its primary and all key secondary objectives, assessing its experimental application in adults with moderate-to-severe conditions. In the trial, Dupixent demonstrated that patients had a fivefold increase in achieving sustained disease remission compared to those receiving a placebo. Sustained disease remission was described as full clinical remission with oral corticosteroids (OCS) tapering completed by week 16, with no relapse and no need for rescue therapy during the 36-week treatment phase.
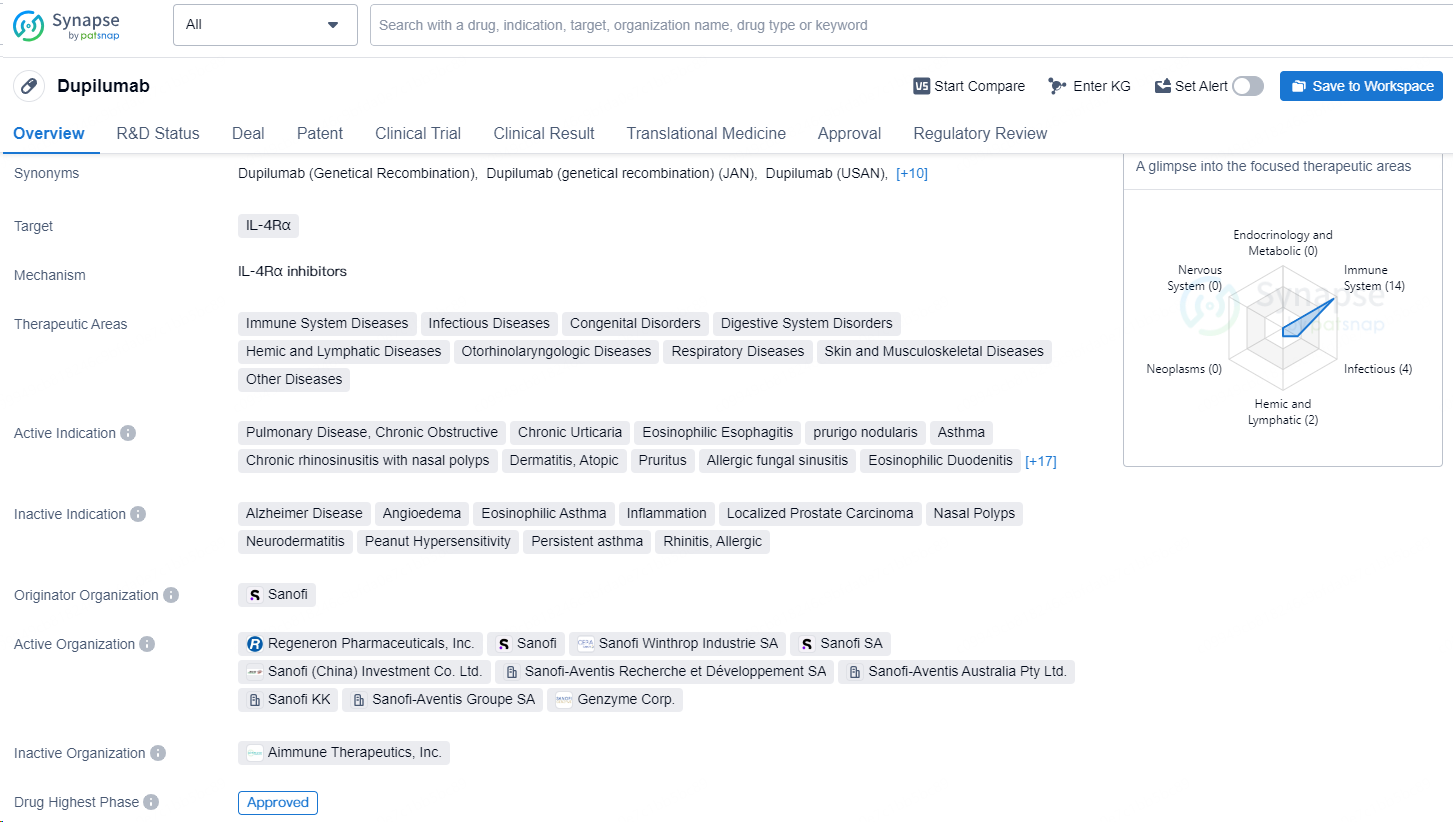
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Dupixent has previously received Orphan Drug Designation from the U.S. Food and Drug Administration (FDA) for bullous pemphigoid (BP), which is an investigational treatment for rare diseases affecting fewer than 200,000 individuals in the United States. This research will contribute to regulatory applications globally, beginning with the United States later this year.
BP is a chronic and relapsing illness characterized by severe itching, blisters, skin reddening, and painful chronic lesions. The blisters and rashes may cover extensive areas of the body, leading to skin bleeding and crusting, which can increase the risk of infection and inhibit daily activities.
Dietmar Berger, M.D., Ph.D., Chief Medical Officer and Global Head of Development at Sanofi, commented:
"The blisters and itching associated with BP can be extremely debilitating, particularly for older patients. There is a considerable unmet medical need for new treatments for this challenging-to-manage disease, where the current standard care involves oral and topical corticosteroids along with immunosuppressants. These treatments often yield poor clinical results and have safety issues, necessitating cautious use in elderly patients. These promising pivotal findings for BP add to a substantial body of scientific evidence highlighting the critical role of IL4 and IL13 in diseases characterized by itching. Combined with the consistent safety profile observed in other dermatological conditions, these results indicate the potential of Dupixent to significantly improve the treatment landscape for BP."
In the ADEPT study, 106 adults with moderate-to-severe BP were assigned randomly to receive either Dupixent 300 mg (n=53) every two weeks following an initial loading dose or a placebo (n=53), along with standard-of-care oral corticosteroids (OCS). Throughout the treatment period, all participants adhered to a protocol-defined tapering regimen for OCS if disease control was maintained.
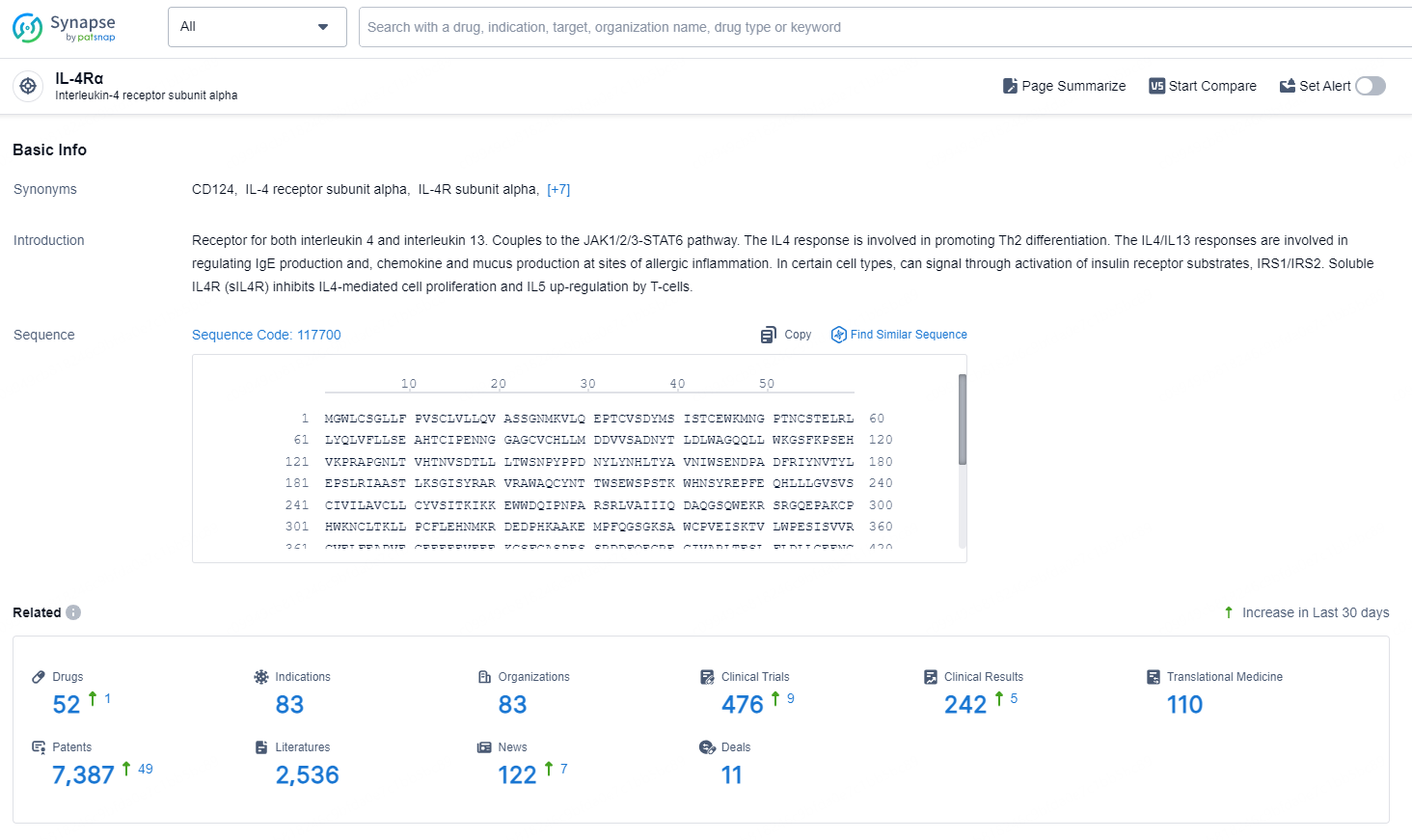
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 14, 2024, there are 52 investigational drugs for the IL-4Rα targets, including 83 indications, 83 R&D institutions involved, with related clinical trials reaching 476, and as many as 7387 patents.
Dupilumab is a monoclonal antibody drug that targets the IL-4Rα and is indicated for various therapeutic areas including immune system diseases, infectious diseases, congenital disorders, digestive system disorders, hemic and lymphatic diseases, otorhinolaryngologic diseases, respiratory diseases, skin and musculoskeletal diseases, and other diseases. The drug is used in the treatment of a wide range of conditions such as pulmonary disease, chronic obstructive pulmonary disease, chronic urticaria, eosinophilic esophagitis, prurigo nodularis, asthma, chronic rhinosinusitis with nasal polyps, atopic dermatitis, pruritus, allergic fungal sinusitis, eosinophilic duodenitis, eosinophilic enteropathy, chronic sinusitis, pemphigoid, bullous, hypersensitivity, allergic asthma, house dust mite allergy, alopecia areata, keloid, colitis (ulcerative), milk hypersensitivity, respiratory disorders, aspergillosis, allergic bronchopulmonary aspergillosis, chronic liver disease, chronic eczema, urticaria, and food hypersensitivity.