CSL Unveils Phase 3 AEGIS-II Results on CSL112 (Apolipoprotein A-I) Efficacy and Safety
Leading biotechnology company CSL has disclosed preliminary findings from the critical Phase 3 AEGIS-II study. This research assessed the performance and security of CSL112 in comparison with a control placebo. Its purpose was to observe the effectiveness in diminishing the likelihood of significant cardiovascular complications in individuals who have recently suffered from an acute myocardial infarction.
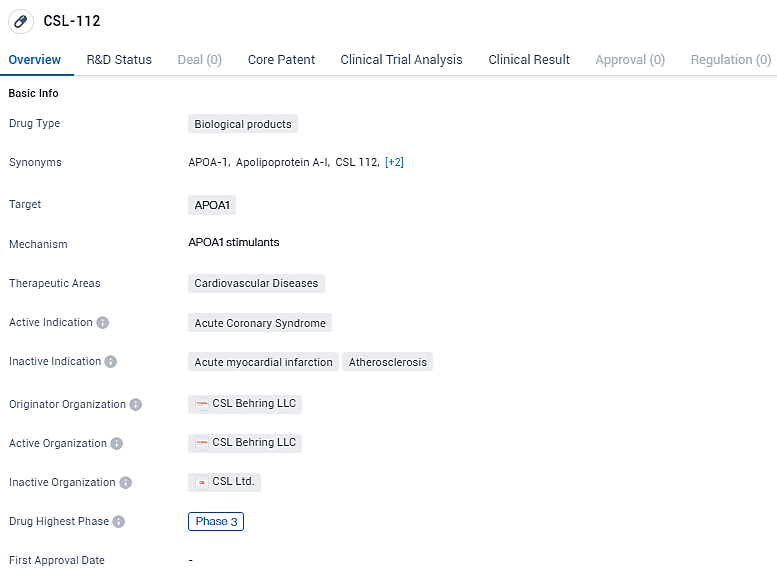
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The research did not achieve its main effectiveness goal of lowering the incidence of major adverse cardiovascular events (MACE) within 90 days. Consequently, no immediate regulatory submission is anticipated. However, CSL112 demonstrated no significant safety or tolerability issues.
"Our team is eager to exchange vital insights we've gained regarding the role of cholesterol efflux in repeated cardiovascular incidents," commented C. Michael Gibson, M.S., M.D., from the Baim Institute for Clinical Research at Harvard Medical School. "Our effort now focuses on a thorough assessment of our data, which we anticipate disclosing in detail shortly."
Ongoing examination of the AEGIS-II trial persists, with the intention to unveil the initial findings at the forthcoming American College of Cardiology Scientific Sessions scheduled for April 6, 2024, followed by a publication in a scholarly journal.
"Deciphering the comprehensive data set and charting the future course for this investigational entity requires significant endeavor. We extend our gratitude to the participants, their families, healthcare professionals, and researchers involved in the AEGIS initiative," expressed Dr. Bill Mezzanotte, the Executive Vice President and Head of Research and Development at CSL.
Dr. Bill Mezzanotte added, "AEGIS-II represents the most extensive exploration in our organization's record, and we take pride in the high-caliber research executed and the advancements we've achieved in research capabilities. These enhancements and our expertise in plasma proteins will be directed towards addressing emergent health concerns in the fields of cardiovascular and metabolic diseases, as well as other key areas of therapeutics we're committed to."
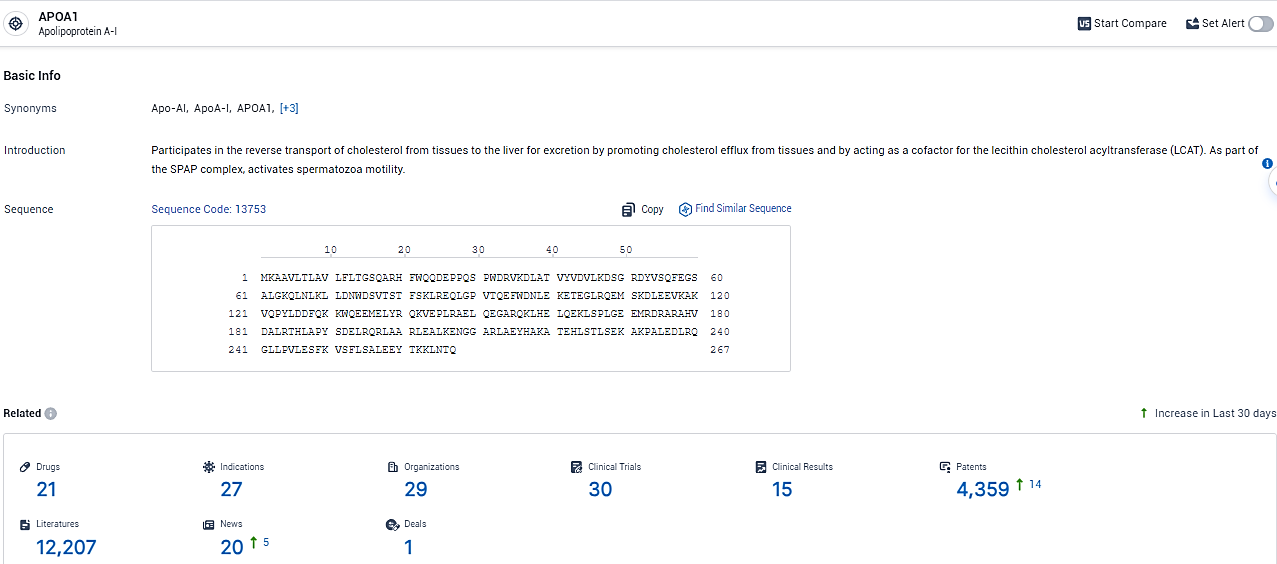
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 19, 2024, there are 21 investigational drugs for the APOA1 target, including 27 indications, 29 R&D institutions involved, with related clinical trials reaching 30, and as many as 4359 patents.
CSL112, is an investigational cholesterol efflux enhancer, developed using a novel formulation of human plasma-derived APOA1 the primary functional component of high-density lipoproteins. Currently in Phase 3 of clinical development, CSL-112 is undergoing rigorous testing to determine its safety and efficacy.