Unveiling Clopidogrel: How to Search for it on Synapse
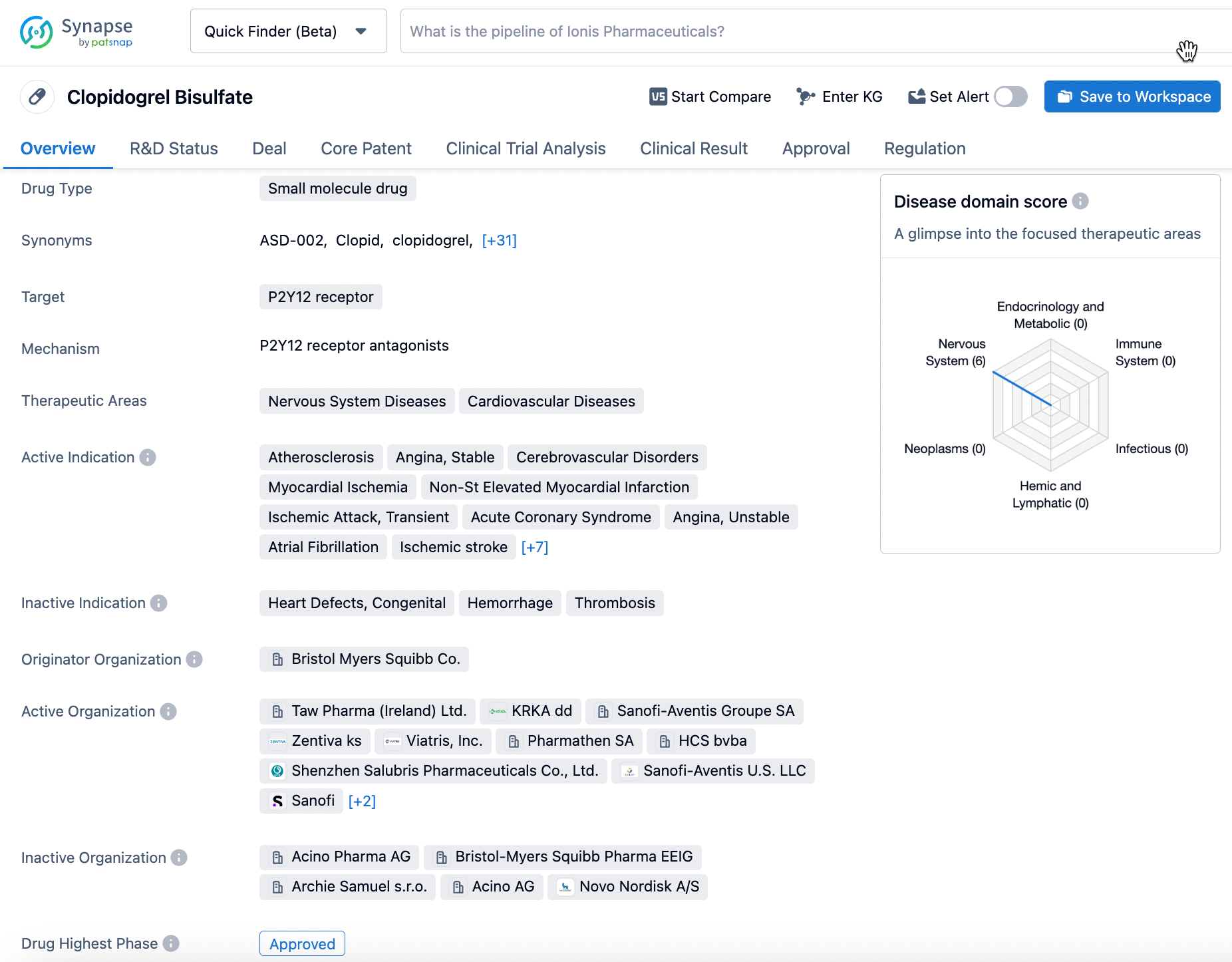
Clopidogrel Bisulfate, a medication trademarked as Plavix®, was approved by Bristol Myers Squibb in the US in 1997. Its mechanism of action is to inhibit P2Y12 receptors, which reduces platelet activation and aggregation. This subsequently decreases the risk of blood clots and their potentially fatal consequences. Clopidogrel is indicated for treating myocardial infarction, stroke, peripheral vascular disease, coronary artery disease, and cerebrovascular disease. With its role as an antiplatelet agent, Clopidogrel proves invaluable in preventing severe complications from blood clots such as heart attacks, strokes, and even death. The drug has shown efficacy in the management and prevention of cardiovascular events, making it an essential medication in modern medicine. Click on the image below to begin the exploration journey of Clopidogrel through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!