CStone Submits Australian Trial Application for Novel Antibody CS2009
CStone Pharmaceuticals (HKEX: 2616), a biopharmaceutical firm focused on innovation, has revealed the submission of a clinical trial application in Australia for CS2009 (a PD-1/VEGF/CTLA-4 trispecific antibody), which is a key asset from the Company’s Pipeline 2.0 designed to treat a variety of solid tumors. This initial human study has been registered and made public on Clinicaltrials.gov (NCT number: NCT06741644).
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
CS2009 incorporates a novel molecular architecture designed to target PD-1, VEGFA, and CTLA-4 simultaneously, while maintaining a balanced affinity for both PD-1 and CTLA-4. This innovative design facilitates a targeted approach towards double-positive tumor-infiltrating T lymphocytes (TILs), effectively inhibiting both PD-1 and CTLA-4 while preserving CTLA-4 activity in single-positive cells. Such a strategy has the potential to minimize systemic toxicity without affecting efficacy. Furthermore, CS2009 promotes rapid and robust internalization, which leads to a reduction in PD-1 and CTLA-4 levels on the TIL membrane. Importantly, CS2009 maintains its full VEGF inhibitory capability, and preclinical findings suggest that its anti-VEGF properties exhibit significant synergy with its immune checkpoint inhibition-crosslinking with VEGFA substantially enhances the actions of both anti-PD-1 and anti-CTLA-4 therapies.
During the 39th Annual Meeting of the Society for Immunotherapy of Cancer (SITC Annual Meeting) in 2024, CStone showcased compelling preclinical results for CS2009, revealing its superior anti-tumor performance relative to potential competitors. These results emphasized CS2009’s capability in targeting a variety of tumor types such as non-small cell lung cancer, ovarian cancer, renal cell carcinoma, cervical cancer, hepatocellular carcinoma, and gastric cancer. CS2009 is poised to emerge as a leading candidate for a first-in-class or best-in-class next-generation immuno-oncology framework.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
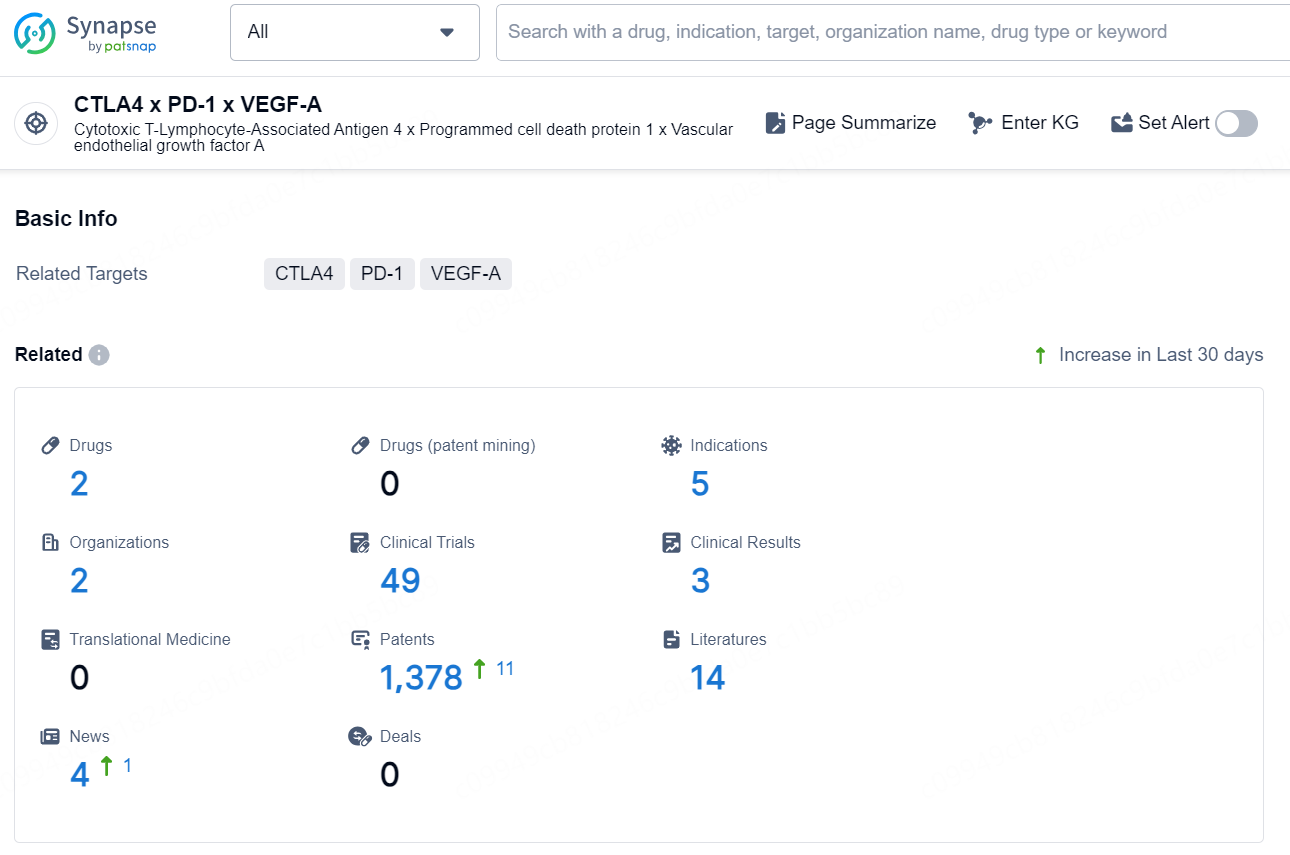
According to the data provided by the Synapse Chemical, As of December 30, 2024, there are 2 investigational drugs for the CTLA4 x PD-1 x VEGF-A target, including 5 indications, 2 R&D institutions involved, with related clinical trials reaching 49, and as many as 1378 patents.
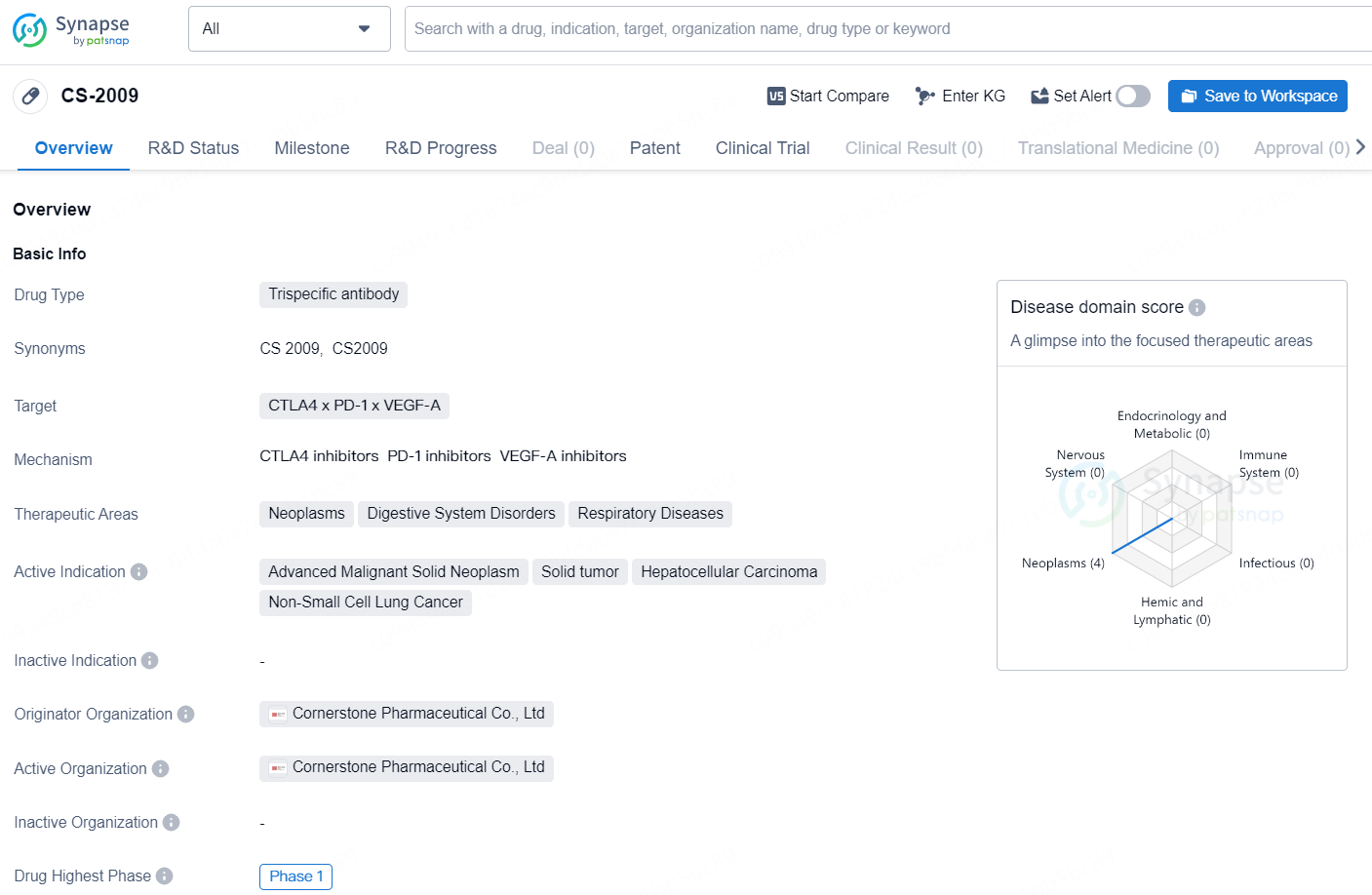
The drug CS-2009 is a trispecific antibody that targets CTLA4, PD-1, and VEGF-A. It is designed to treat neoplasms, digestive system disorders, and respiratory diseases. The active indications for CS-2009 include advanced malignant solid neoplasm, solid tumor, hepatocellular carcinoma, and non-small cell lung cancer. The drug is developed by Cornerstone Pharmaceutical Co., Ltd.