RAD 202 Gets Green Light to Begin Phase 1 Therapy Trial
Radiopharm Theranostics, a biopharmaceutical entity in the clinical stage concentrating on the advancement of novel oncology radiopharmaceuticals to address significant unmet medical needs, is delighted to report that it has received approval from the Belberry Human Research Ethics Committee (HREC) in Australia. This approval allows the company to commence its First-In-Human (FIH) Phase 1 therapeutic clinical trial of 177Lu-labelled RAD 202 aimed at treating HER2-expressing solid tumors.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The open-label Phase 1 study, named ‘HEAT’ (HER2 Antibody Therapy with Lutetium-177), is being conducted to explore the dose escalation of 177Lu-RAD202. The aim is to assess the safety and initial clinical efficacy of this innovative radiotherapeutic approach in patients suffering from advanced cancers that express HER2. RAD 202 is classified as a single-domain monoclonal antibody (sdAb) that specifically targets the Human Epidermal Growth Factor Receptor 2 (HER2), a marker that is frequently overexpressed in breast cancer and various other solid tumors, making it a well-established target in cancer treatment. This multicenter trial is set to enroll participants across Australia, facilitated by the prominent oncology care organization GenesisCare CRO.
Prior research has shown the safety profile and biodistribution of 99mTc-labeled RAD 202 in human subjects. Furthermore, recent preclinical studies with 177Lu-labeled RAD 2022 have indicated its therapeutic benefits in HER2-positive xenografts. These findings revealed substantial tumor growth inhibition and significantly extended survival durations, reinforcing the rationale for first-in-human (FIH) dose-finding investigations.
“We are excited to have received the green light to move forward with our Phase 1 FIH basket trial in Australia,” commented Riccardo Canevari, CEO and Managing Director of Radiopharm Theranostics. “RAD 202 has the potential to fill a significant therapeutic void for HER2-positive metastatic patients who are resistant to or unable to benefit from existing standard treatments. Through RAD 202, we aspire to offer an alternative approach that may enhance clinical results for patients with HER2-positive advanced cancers while possibly maintaining their quality of life.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
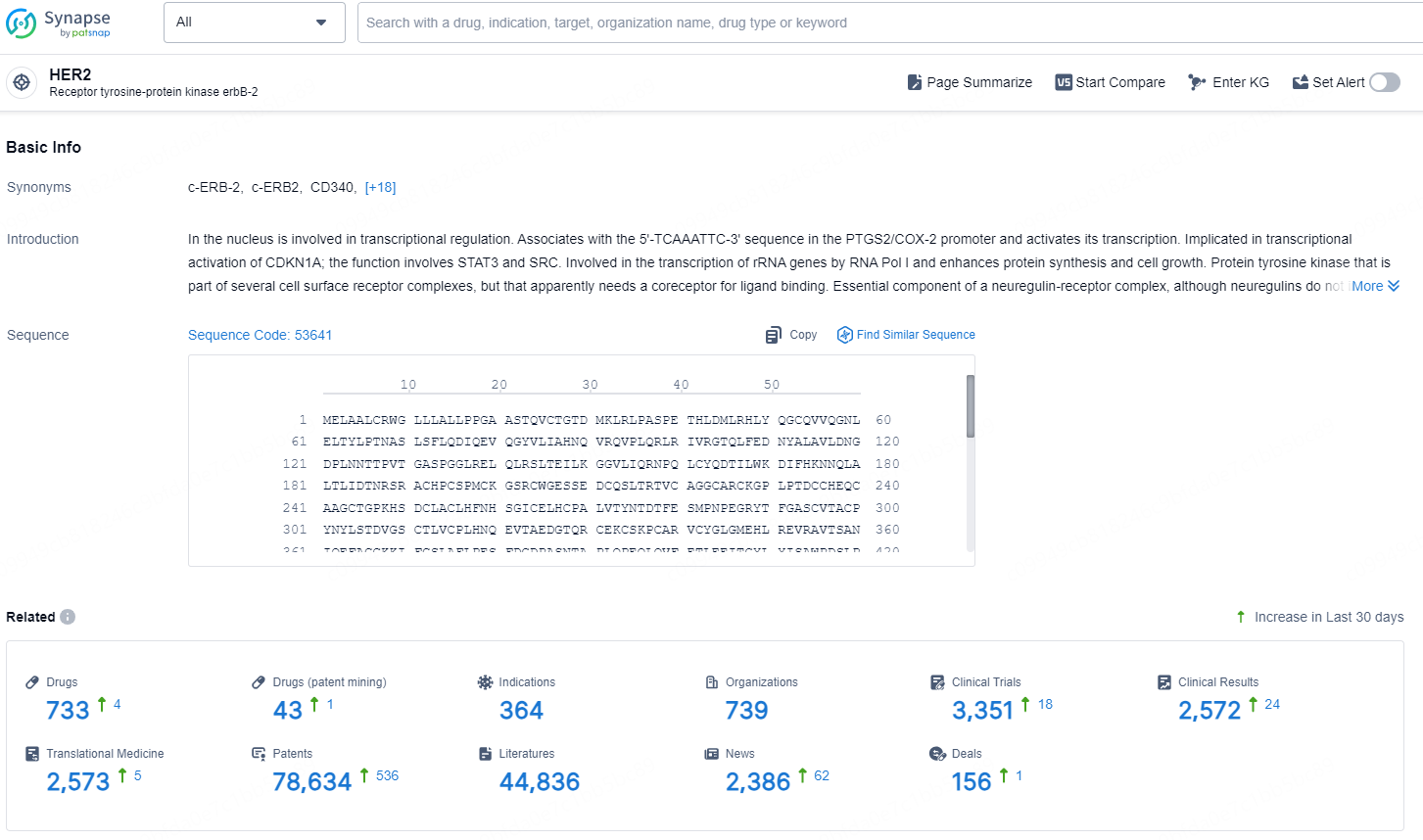
According to the data provided by the Synapse Database, As of December 30, 2024, there are 733 investigational drug for the HER2 targets, including 364 indications, 739 R&D institutions involved, with related clinical trials reaching 3351, and as many as 78634 patents.
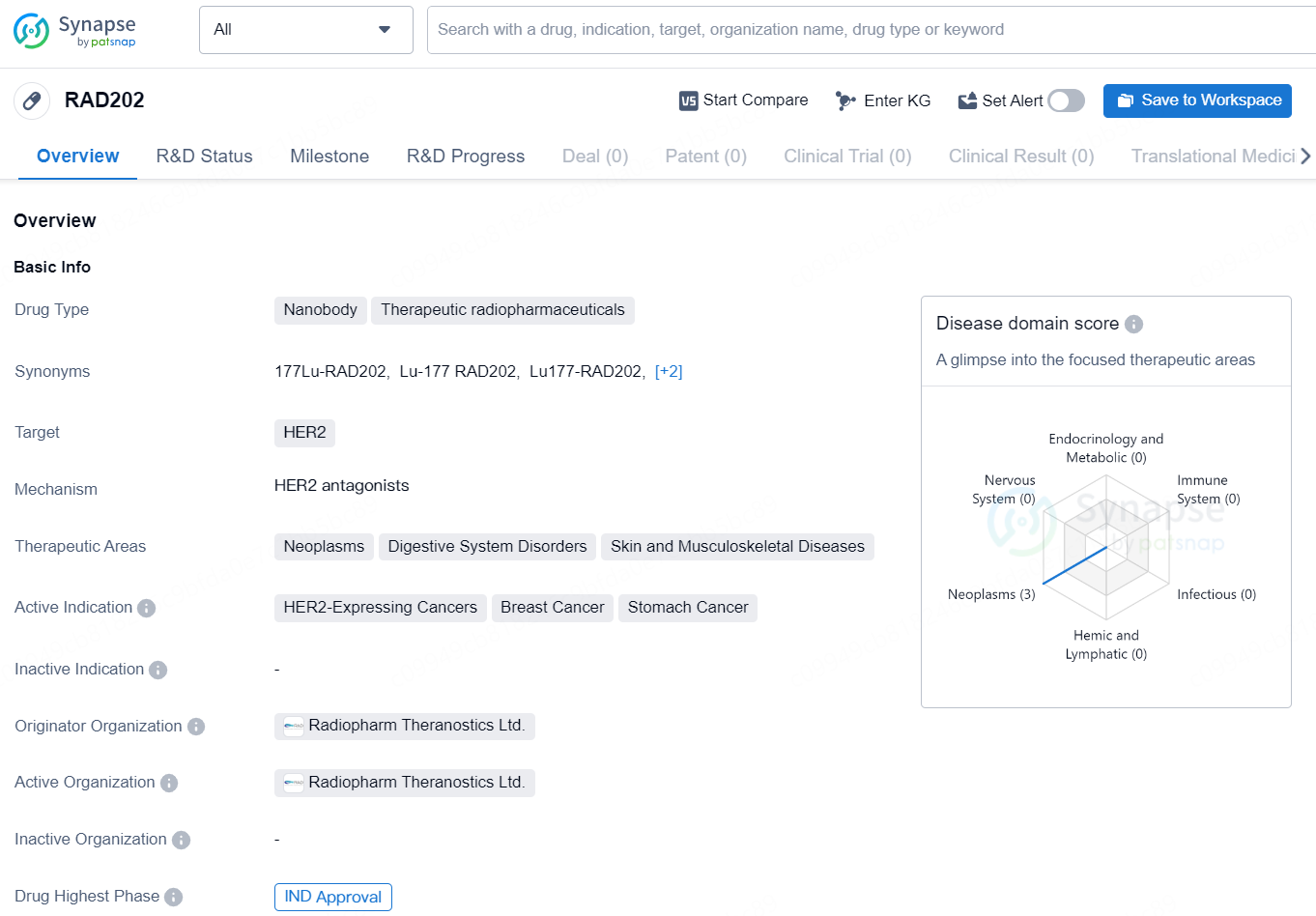
RAD202 is a nanobody-based therapeutic radiopharmaceutical that targets HER2. It is primarily intended for the treatment of neoplasms, digestive system disorders, and skin and musculoskeletal diseases. The active indications for RAD202 include HER2-expressing cancers, breast cancer, and stomach cancer. The drug is developed by Radiopharm Theranostics Ltd., and has received IND (Investigational New Drug) approval, indicating that it is safe to proceed with clinical trials in the United States.