FDA Approves Alhemo® Injection for Hemophilia A/B with Inhibitors in Ages 12+
Novo Nordisk has announced that the U.S. Food and Drug Administration (FDA) has granted approval for Alhemo® (concizumab-mtci) injection as a once-daily preventive treatment aimed at decreasing the incidence of bleeding episodes in both adult and pediatric patients aged 12 and older suffering from hemophilia A or B with inhibitors. This approval marks the continuation of Novo Nordisk's dedication to individuals affected by rare bleeding disorders for over 35 years. Alhemo® functions as a tissue factor pathway inhibitor (TFPI) antagonist and is available in a prefilled, premixed pen for subcutaneous use (offered in doses of 60 mg/1.5 mL, 150 mg/1.5 mL, or 300 mg/3 mL) administered through a separate thin 32-gauge, 4 mm needle. At present, the majority of hemophilia A or B treatments with inhibitors are delivered through intravenous infusions; thus, Alhemo® represents the first subcutaneous injection option designed for this specific patient group.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Alhemo® is engineered to inhibit a protein known as TFPI within the body, which is responsible for preventing blood coagulation. By obstructing TFPI, Alhemo® enhances the generation of thrombin, a protein crucial for promoting blood clotting and reducing bleeding, particularly in situations where other coagulation factors are deficient or impeded by inhibitors.
Approximately 30% of individuals diagnosed with severe hemophilia A and between 5-10% of those with severe hemophilia B develop inhibitors, complicating the treatment process for certain patients significantly. Although advancements have improved the quality of life for many individuals with hemophilia, patients with hemophilia B who have inhibitors continue to face substantial treatment challenges due to the lack of effective preventive options for bleeding. Because of the significant unmet medical requirements in this group and the promising Phase 2 trial outcomes, the FDA awarded Alhemo® Breakthrough Therapy designation for the treatment of hemophilia B with inhibitors.
“The authorization of Alhemo® marks an outstanding milestone in the preventive treatment of hemophilia for patients aged 12 and older with inhibitors, who often face limited options,” remarked Anna Windle, SVP of Clinical Development at Novo Nordisk. “As the first of its kind for this demographic, Alhemo® is a critical advancement in addressing the needs of hemophilia patients with inhibitors, reinforcing Novo Nordisk's dedication to patient-focused innovations in the field of rare diseases.”
The main aim of the pivotal Phase 3 explorer7 trial was to evaluate the number of spontaneous and traumatic bleeding episodes treated, as indicated by the annual bleeding rate (ABR). Results demonstrated an 86% reduction in ABR for patients assigned to receive Alhemo® prophylaxis compared to those without it (ABR ratio of 0.14, 95% confidence interval [CI], 0.07 to 0.29, p-value <0.001). The estimated mean ABR for patients receiving Alhemo® prophylaxis was 1.7, whereas it was 11.8 for those not receiving such treatment, with the overall median ABR at zero for those treated for spontaneous and traumatic bleeding compared to 9.8 in the non-prophylaxis group. Furthermore, as a supportive secondary efficacy measure, 64% of patients given Alhemo® prophylaxis reported no spontaneous or traumatic bleeding episodes during the initial 24 weeks of treatment, in contrast to 11% in the absence of prophylaxis. In the explorer7 study, the most frequently observed adverse effects among ≥5% of patients receiving Alhemo® included injection site reactions (18%) and urticaria (6%). Serious adverse reactions identified included renal infarct and hypersensitivity reactions.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
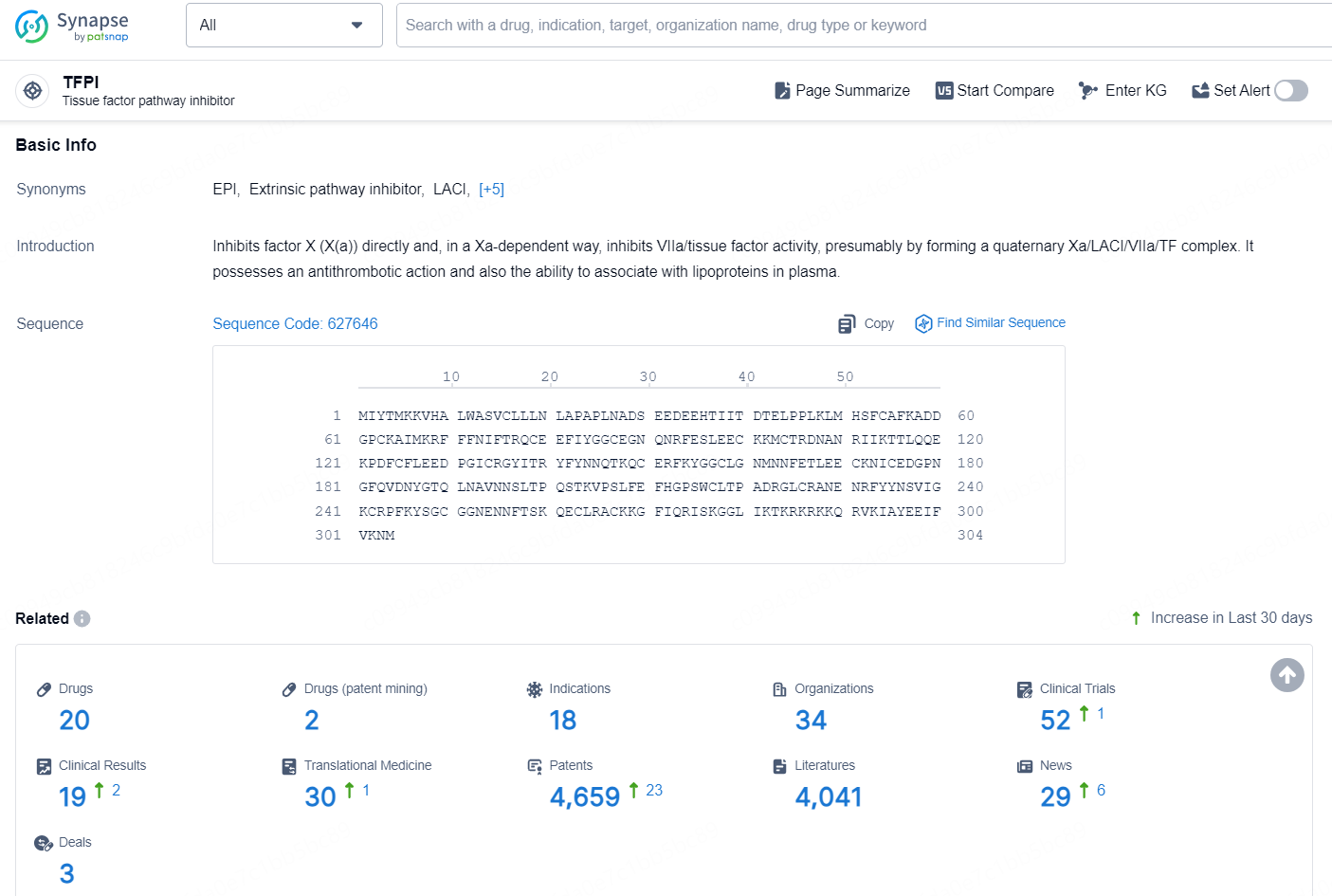
According to the data provided by the Synapse Database, As of December 30, 2024, there are 20 investigational drugs for the TFPI target, including 18 indications, 34 R&D institutions involved, with related clinical trials reaching 52, and as many as 4659 patents.
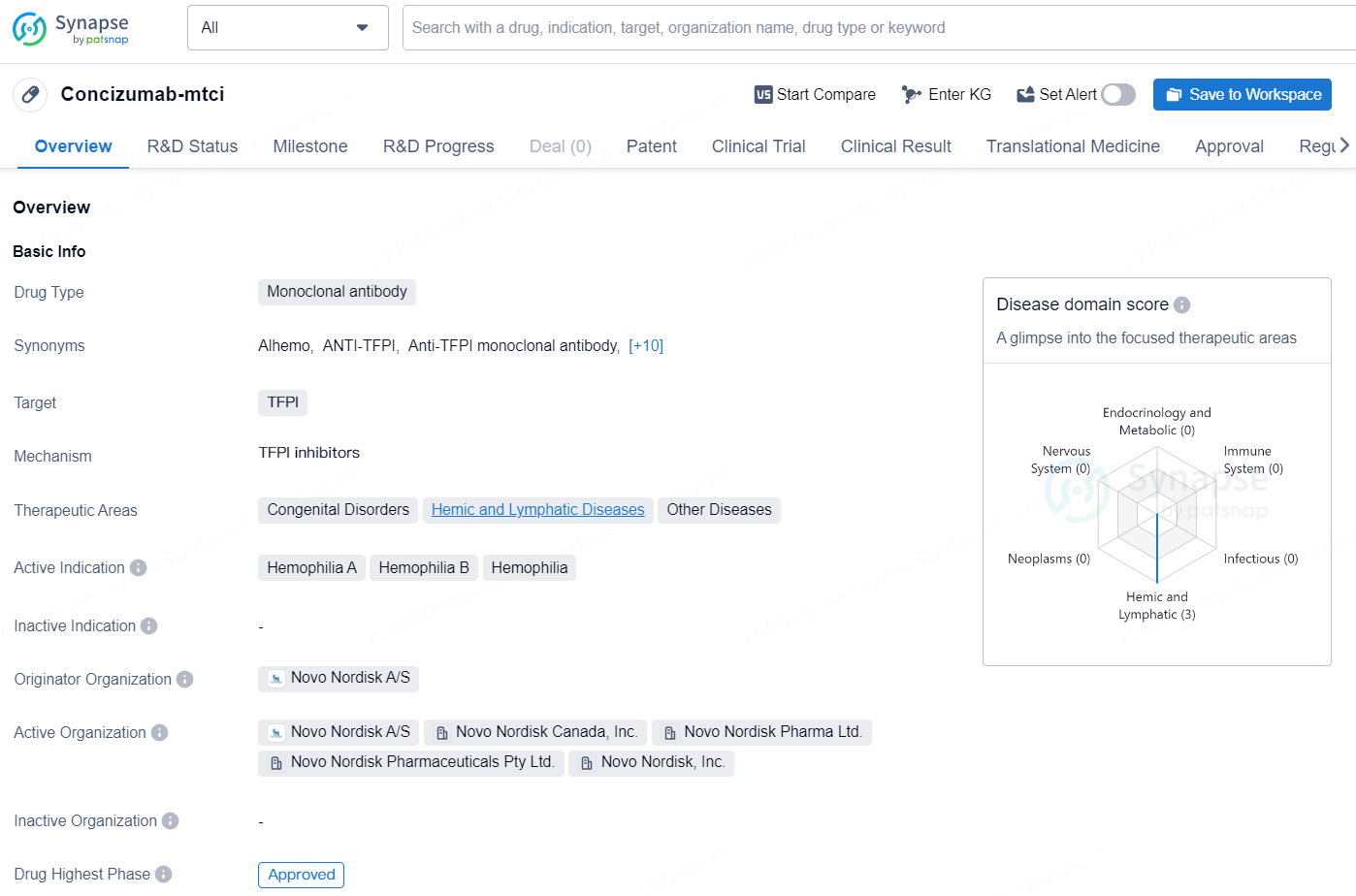
Concizumab-mtci is a monoclonal antibody drug developed by Novo Nordisk A/S, with its highest phase of development being approved for use. The drug targets TFPI and is indicated for use in the therapeutic areas of congenital disorders, hemic and lymphatic diseases, and other diseases, particularly for the treatment of hemophilia A, B, and other forms of hemophilia. The drug received its first approval in Canada in March 2023, and it is regulated as a priority review, orphan drug, and breakthrough therapy.