FDA Approves Pfizer's BRAFTOVI® Combo for First-Line Treatment of BRAF V600E Metastatic Colorectal Cancer
Pfizer Inc. (NYSE: PFE) has announced that the U.S. Food and Drug Administration (FDA) has granted approval for BRAFTOVI® (encorafenib) in conjunction with cetuximab (known commercially as ERBITUX®) and mFOLFOX6 (which includes fluorouracil, leucovorin, and oxaliplatin) for managing metastatic colorectal cancer (mCRC) in patients with a BRAF V600E mutation, as confirmed by an FDA-approved diagnostic test. This approval was based on results demonstrating a statistically significant and clinically relevant increase in both response rate and duration of response among treatment-naïve patients receiving the BRAFTOVI combination as observed in the Phase 3 BREAKWATER trial. The ongoing approval for this treatment pathway hinges on the validation of its clinical benefits. This accelerated approval is noteworthy as it represents one of the pioneering efforts in the industry under the FDA’s Project FrontRunner initiative, aimed at facilitating the advancement and approval of novel cancer therapeutics for advanced or metastatic conditions.
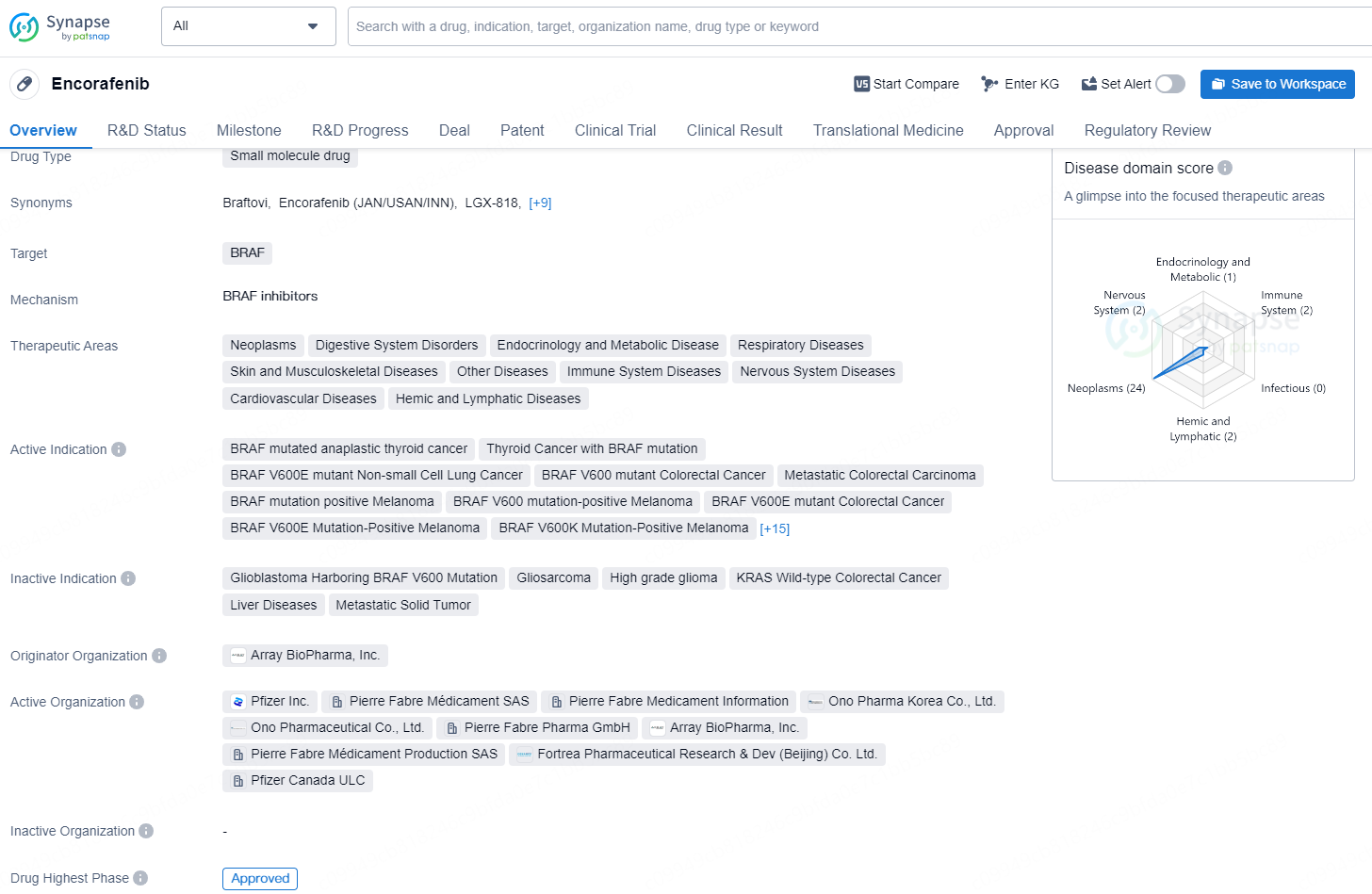
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
“Patients diagnosed with metastatic colorectal cancer harboring BRAF mutations have historically faced restricted treatment options and poor prognoses,” stated Scott Kopetz, M.D., Ph.D., FACP, Professor and Deputy Chair of Gastrointestinal Medical Oncology at The University of Texas MD Anderson Cancer Center and co-principal investigator of the BREAKWATER study. “The encorafenib regimen is the first and only combination therapy that includes a BRAF-targeted agent for this patient group and can be used as a first-line treatment. It has shown high response rates that are both rapid and durable, signifying a promising development in disease management and providing renewed optimism for patients.”
The ongoing BREAKWATER trial assesses BRAFTOVI in combination with cetuximab, with or without chemotherapy (mFOLFOX6), in previously untreated patients with metastatic colorectal cancer who have a BRAF V600E mutation. This Phase 3 study is unique as it investigates a BRAF-targeted regimen for first-line treatment in this specific patient population. It achieved one of its two primary endpoints pertaining to confirmed overall response rate (ORR), demonstrating a statistically significant advantage over standard treatment: the ORR for the combination of BRAFTOVI, cetuximab, and mFOLFOX6 was 61% (95% confidence interval [CI]: 52, 70), compared to 40% (95% CI: 31, 49) for chemotherapy, with or without bevacizumab (p=0.0008).1 The median duration of response (DoR) for the BRAFTOVI combination was 13.9 months (95% CI: 8.5, not estimable), compared to 11.1 months (95% CI: 6.7, 12.7) for the chemotherapy regimen.1 The Phase 3 BREAKWATER trial is currently ongoing, and results from the complete dataset will be shared in future medical meetings.
“For over ten years, Pfizer has been at the forefront of providing targeted therapies for cancers driven by molecular factors. With the recent accelerated approval of the BRAFTOVI regimen, individuals with BRAF V600E mutant metastatic colorectal cancer now have access to a first-line treatment option that specifically targets the mutation contributing to their disease,” remarked Chris Boshoff, M.D., Ph.D., Chief Oncology Officer and Executive Vice President at Pfizer. “This achievement enhances our legacy of innovating treatments for BRAF-driven tumors, which are some of the most challenging cancers to manage. We are eager to continue broadening our portfolio, including the development of next-generation brain-penetrant BRAF inhibitors.”
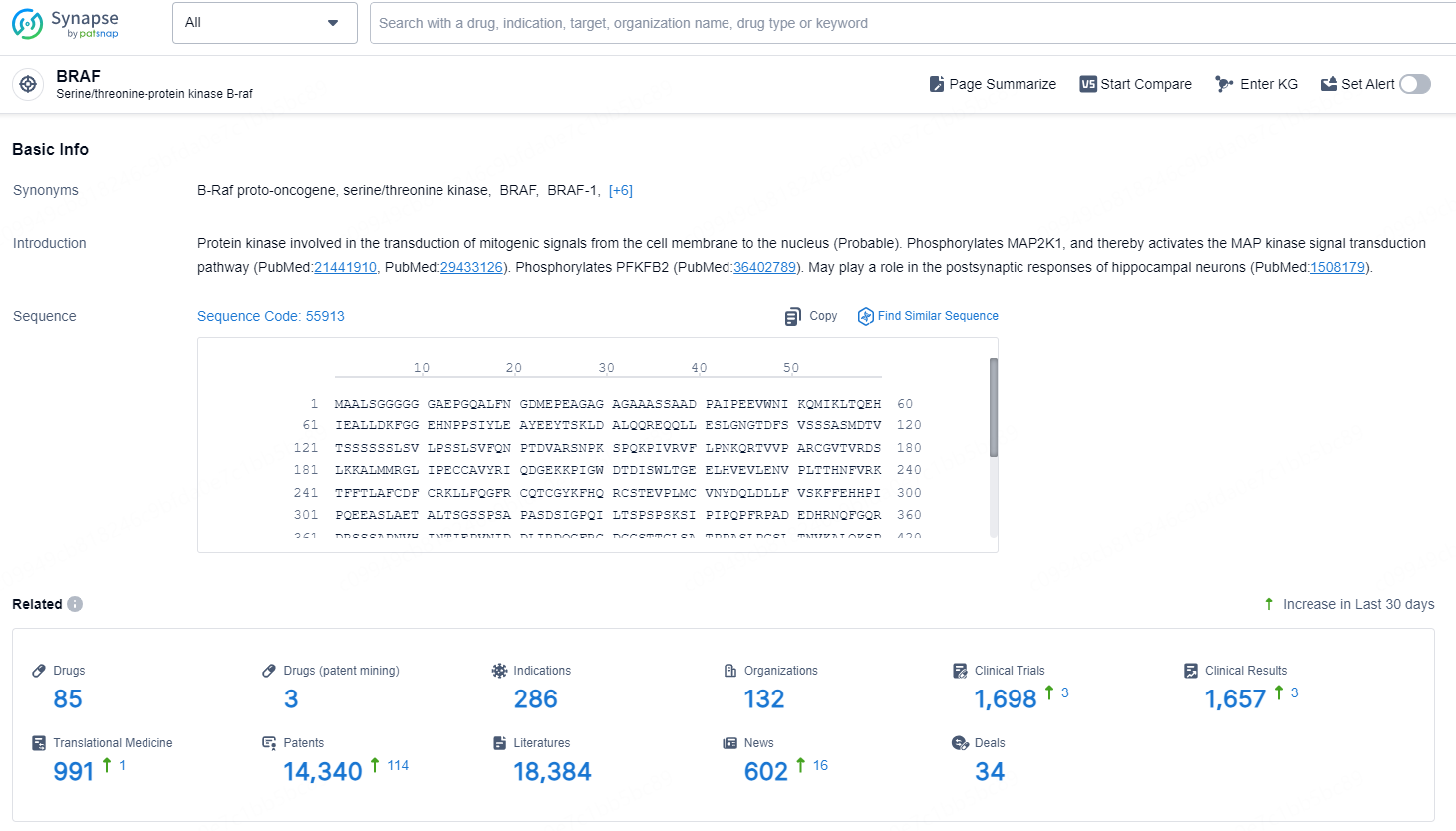
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 30, 2024, there are 85 investigational drugs for the BRAF target, including 286 indications, 132 R&D institutions involved, with related clinical trials reaching 1698 and as many as 14340 patents.
Encorafenib is a targeted therapy drug primarily used in the treatment of certain types of cancer, especially melanoma. It is a BRAF inhibitor, meaning it targets and inhibits the BRAF protein, which is part of a signaling pathway that regulates cell growth. Mutations in the BRAF gene, particularly the BRAF V600E mutation, are commonly found in various cancers, including melanoma, and contribute to uncontrolled cancer cell growth.