NPMA Accepts Second NDA for Kelun-Biotech’s SKB264 in EGFR-Mutant NSCLC
The Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) of China has accepted Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (“Kelun-Biotech”, 6990.HK)'s new drug application (NDA) for its core product, sacituzumab tirumotecan (sac-TMT, formerly SKB264/MK-2870). This acceptance was based on the positive outcomes from the pivotal OptiTROP-Lung03 study.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
OptiTROP-Lung03, a crucial, multi-center, randomized clinical trial, is assessing the efficacy of sac-TMT monotherapy administered intravenously at a dose of 5mg/kg every other week (Q2W) against docetaxel for patients suffering from locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) mutations, who have not responded to EGFR-tyrosine kinase inhibitor (TKI) treatment and platinum-based chemotherapy. During a predefined analysis, sac-TMT monotherapy showed a statistically significant and clinically meaningful enhancement in both objective response rate (ORR) and progression-free survival (PFS) compared with docetaxel.
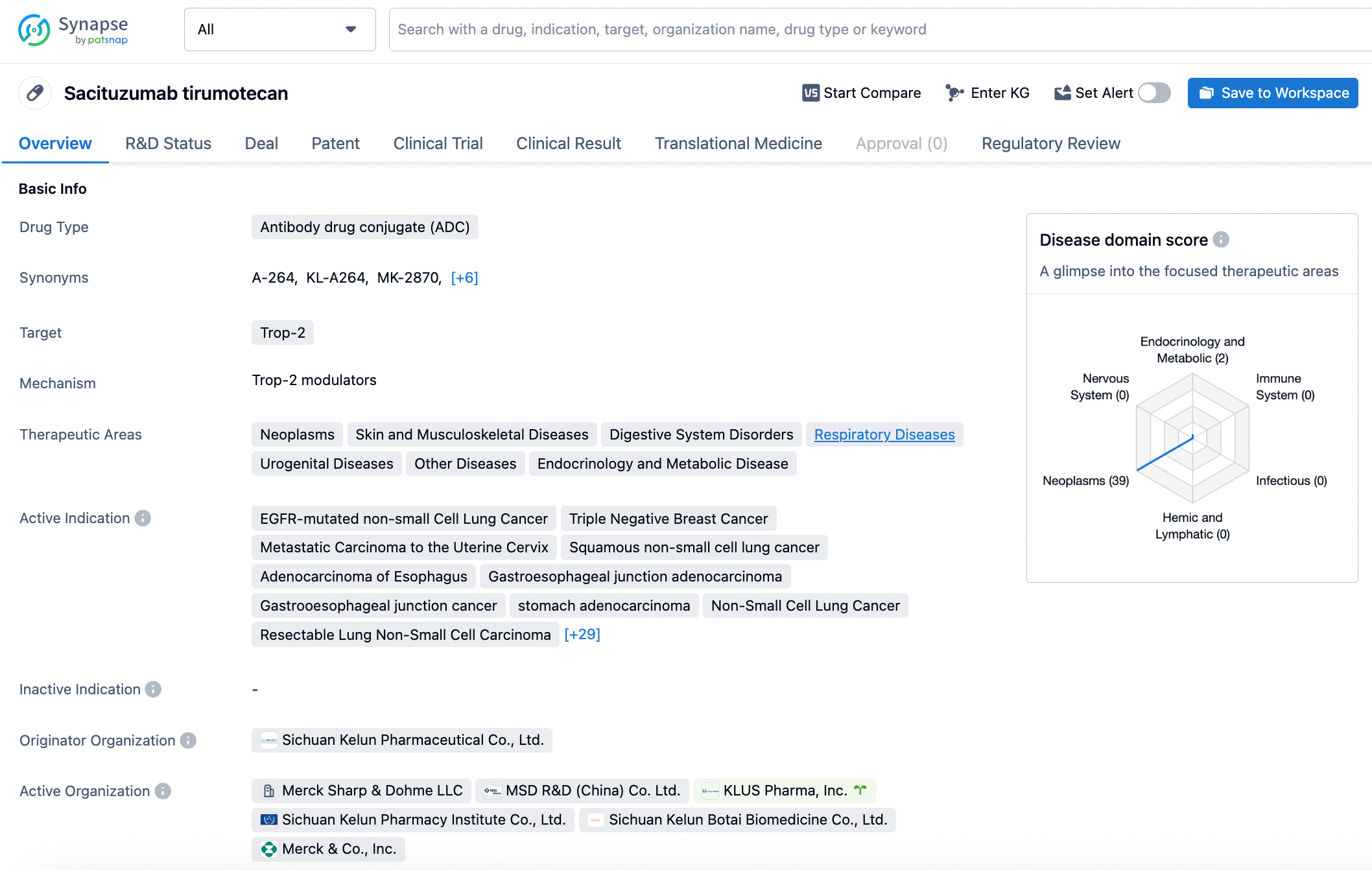
Lung cancer primarily consists of non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), with NSCLC being the predominant type, encompassing approximately 80%-85% of lung cancers. The molecular characteristics of NSCLC in China differ from those in Western populations, with EGFR mutations being a common gene variant found in around 40%-50% of lung adenocarcinoma patients in China. According to the 2024 CSCO guidelines, EGFR-TKIs are the recommended treatment for stage IV EGFR-mutant NSCLC; following resistance to EGFR-TKIs, platinum-based chemotherapy is the primary first-line treatment. Current treatment options are ineffective for patients who do not respond to EGFR-TKIs and platinum-based chemotherapy.
Single-agent chemotherapy, particularly docetaxel, is the current standard for these patients, with an ORR of 3.2%-10.8%, a median PFS of about 2 months, and a median OS of approximately 6-8 months. There is a significant unmet medical need for new treatments to improve survival in patients with locally advanced or metastatic EGFR-mutated NSCLC who have failed previous therapies with EGFR-TKIs and platinum-based chemotherapy.
Kelun-Biotech has submitted an application for the use of sac-TMT for injection in the treatment of patients with locally advanced or metastatic EGFR-mutant NSCLC who have not responded to previous EGFR-TKI and platinum-based chemotherapy treatments.
This application is the second New Drug Application (NDA) for sac-TMT that has been accepted by the NMPA. The official CDE website announced on August 14, 2024, that this application would be included in the priority review and approval process. Previously, an NDA for sac-TMT in patients with locally advanced or metastatic triple-negative breast cancer (TNBC) who had received at least two prior systemic treatments (including at least one for the advanced or metastatic setting) was accepted by the NMPA.
Dr. Junyou Ge, CEO of Kelun-Biotech, expressed his honor in having the second NDA for SKB264 accepted, emphasizing Kelun-Biotech's commitment to an innovation-driven development strategy, exploring advanced technologies and new treatment approaches for major diseases. The company aims to address unmet medical needs with the original development of novel drugs that possess unique advantages and international potential. By enhancing end-to-end capabilities in innovative drug development, the company continuously improves the efficiency and success rates of research and development, prioritizing clinical research progress. Dr. Ge reaffirmed the company's dedication to exploring and validating the clinical value of key projects rapidly, guided by a commitment to excellence and global oncology health.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
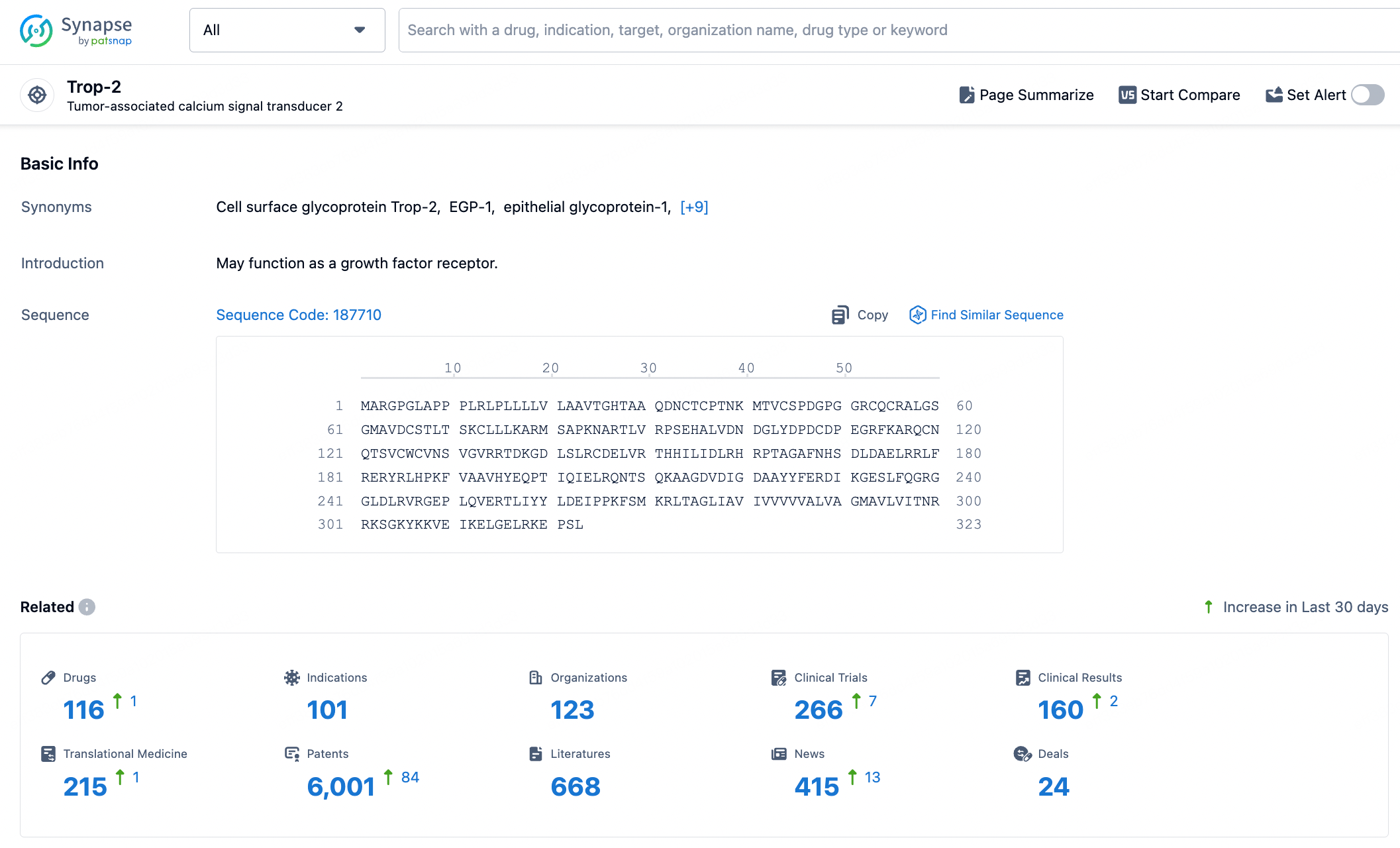
According to the data provided by the Synapse Database, As of August 23, 2024, there are 4 investigational drugs for the Trop-2 targets, including 101 indications, 123 R&D institutions involved, with related clinical trials reaching 266, and as many as 6001 patents.
Sacituzumab tirumotecan is an antibody drug conjugate (ADC) that targets the Trop-2 protein. It is being developed for the treatment of a wide range of therapeutic areas, including neoplasms, skin and musculoskeletal diseases, digestive system disorders, respiratory diseases, urogenital diseases, endocrinology and metabolic diseases, and more.