IGC Pharma Expands Drug Lineup with Potential GLP-1 Agonist IGC-1A for Metabolic Diseases
IGC Pharma, Inc. (NYSE American: IGC) (“IGC Pharma” or the “Company”) announced today that its unique molecule, IGC-1A, has been recognized as a prospective GLP-1 agonist through advanced AI modeling techniques utilized by the Company. This breakthrough underscores the flexibility of IGC Pharma’s drug development platforms and indicates the Company’s potential to strategically extend its reach into metabolic disease treatment and weight management.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Identifying IGC-1A as a potential GLP-1 agonist represents a crucial milestone for IGC Pharma, highlighting a significant market opportunity and enhancing patient care for various medical conditions. GLP-1 (glucagon-like peptide-1) is a potent hormone that plays a key role in controlling blood glucose levels and aiding weight loss. GLP-1 agonists are gaining popularity for their effectiveness in treating type 2 diabetes and supporting weight management by enhancing satiety and decreasing appetite. Additionally, they are being investigated for their neuroprotective properties, which could potentially transform treatments for Alzheimer’s disease by mitigating inflammation and oxidative stress, thereby improving patient outcomes in a comprehensive manner.
Ram Mukunda, CEO of IGC Pharma, stated, “Our AI-driven research has identified a promising potential for IGC-1A and IGC-1C to act as GLP-1 agonists. This breakthrough bolsters our Alzheimer’s treatment offerings and also positions us well to enter the booming weight loss market. Our AI model suggests that IGC-1A and IGC-1C could be effective in treating metabolic disorders when compared to well-known drugs like Ozempic, Tirzepatide, Retatrutide, and Metformin. Although we are at an early stage, these molecules provide us with the potential to develop a portfolio targeting metabolic disorders and obesity, with better tolerability and efficacy than existing treatments."
"As we move toward further validation in clinical trials, we anticipate substantial growth in creating innovative treatments for metabolic and neurodegenerative diseases. Further studies on IGC-1A have disclosed its potential roles as a GLP-1 agonist, GIP agonist, and a CB1r inverse agonist, paving the way for potentially significant therapies in weight management, neurological conditions, and metabolic disorders."
In 2024, IGC Pharma plans to advance toxicology and dosing studies to prepare for filing an Investigational New Drug Application for IGC-1A with the FDA, although this is not guaranteed. The potential strategic expansion of IGC Pharma into metabolic disorders demonstrates the Company’s dedication to utilizing advanced AI technology to foster innovation and develop impactful solutions, thereby creating substantial value for investors.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
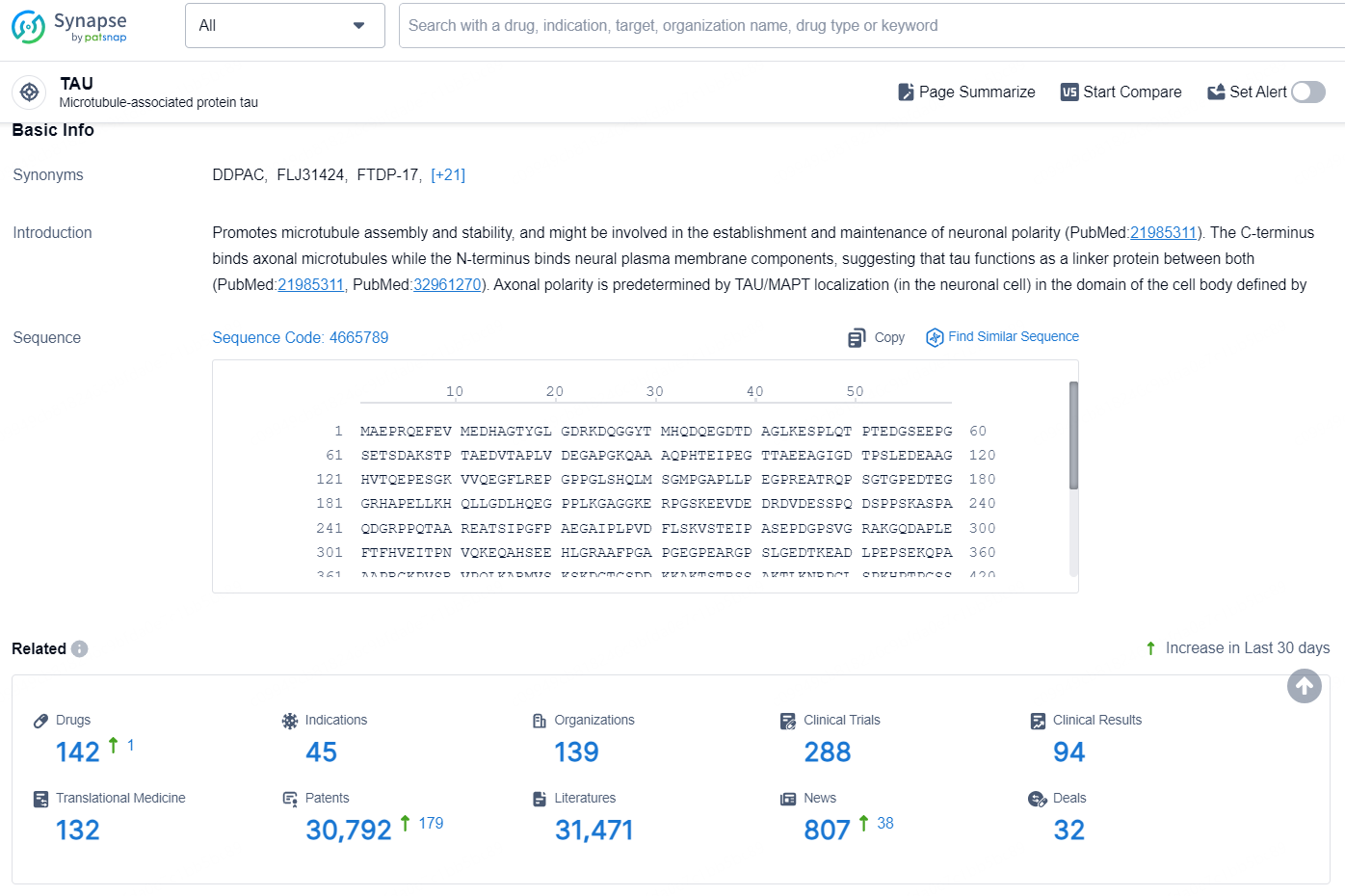
According to the data provided by the Synapse Database, As of August 23, 2024, there are 142 investigational drugs for the TAU targets, including 45 indications, 139 R&D institutions involved, with related clinical trials reaching 288, and as many as 30792 patents.
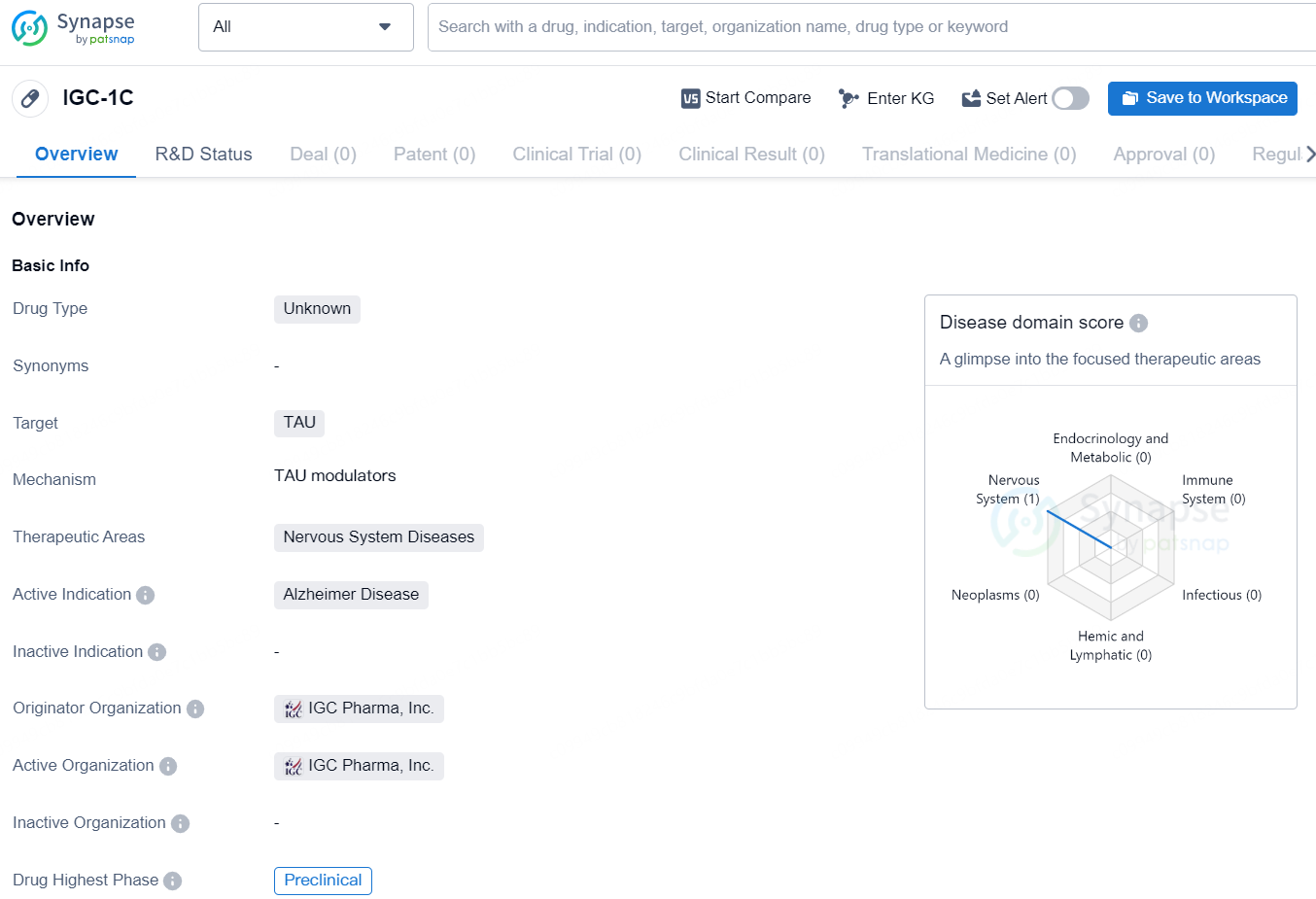
IGC-1C is a drug of unknown type that is being developed by IGC Pharma, Inc. The drug targets TAU, a protein associated with neurodegenerative diseases, particularly Alzheimer's Disease. The therapeutic areas of focus for IGC-1C are nervous system diseases, with a specific focus on Alzheimer's Disease. Currently, the drug is in the preclinical phase, indicating that it has not yet advanced to human trials and is still being tested in laboratory and animal studies.