Deciphering Telomerase Inhibitors and Keeping Up with Their Recent Developments
Telomerase is an enzyme that plays a crucial role in maintaining the integrity and stability of our DNA. It is responsible for the maintenance and elongation of telomeres, which are protective caps at the ends of our chromosomes. Telomeres shorten with each cell division, eventually leading to cellular aging and senescence. Telomerase counteracts this process by adding repetitive DNA sequences to the telomeres, preventing their degradation and ensuring the replication of genetic material. However, telomerase activity is tightly regulated in normal cells, as its overexpression can lead to uncontrolled cell growth and cancer. Understanding telomerase and its regulation is essential for developing therapies targeting aging and cancer.
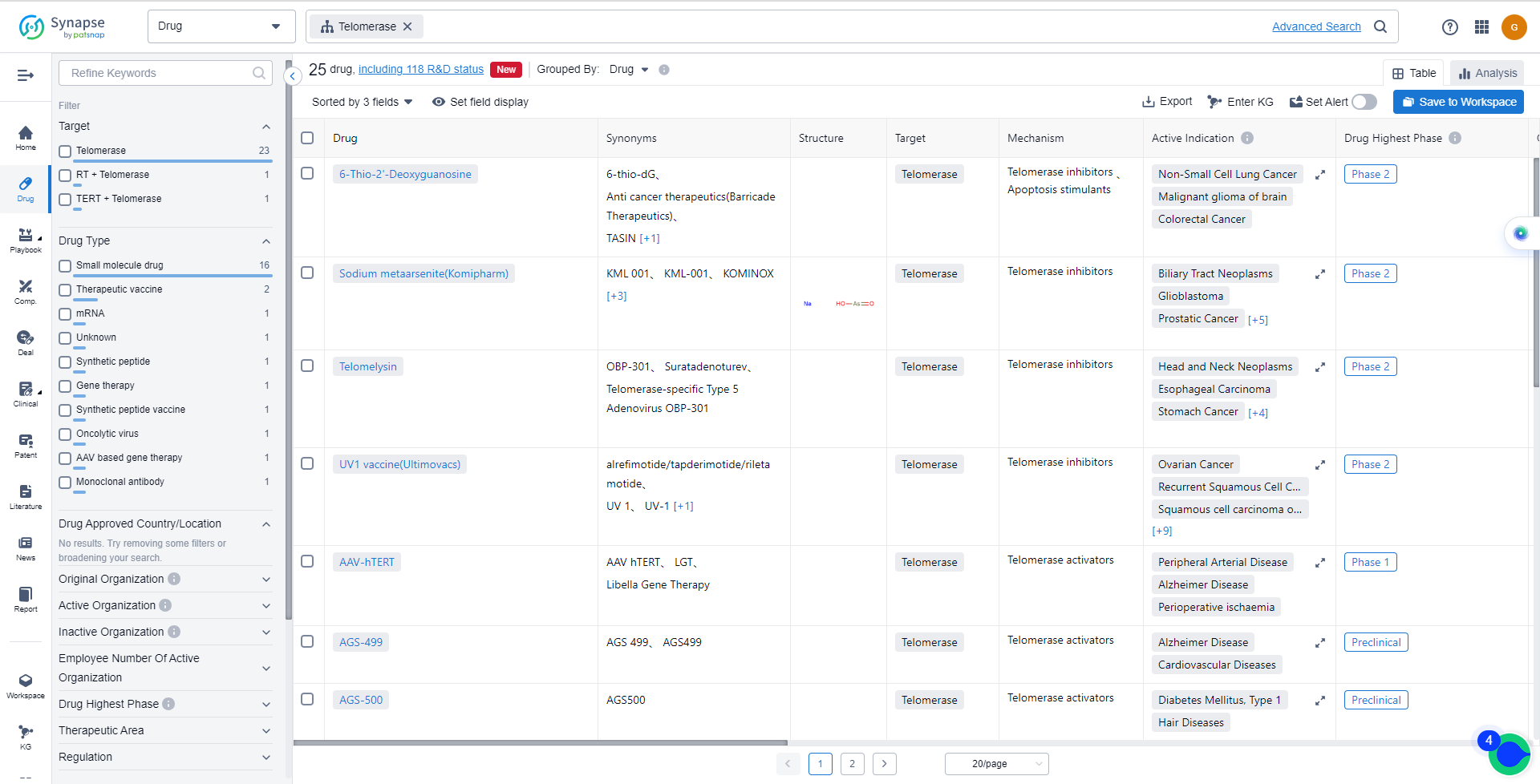
The current competitive landscape of target Telomerase is characterized by multiple companies actively pursuing drug development, particularly in Phase 2. Komipharm International Co., Ltd., Oncolys BioPharma, Inc., MAIA Biotechnology, Inc., Medigen Biotechnology Corp., and Ultimovacs ASA are some of the companies with drugs in Phase 2, indicating their advanced R&D progress. However, no drugs under the target Telomerase have been approved for relevant indications yet.
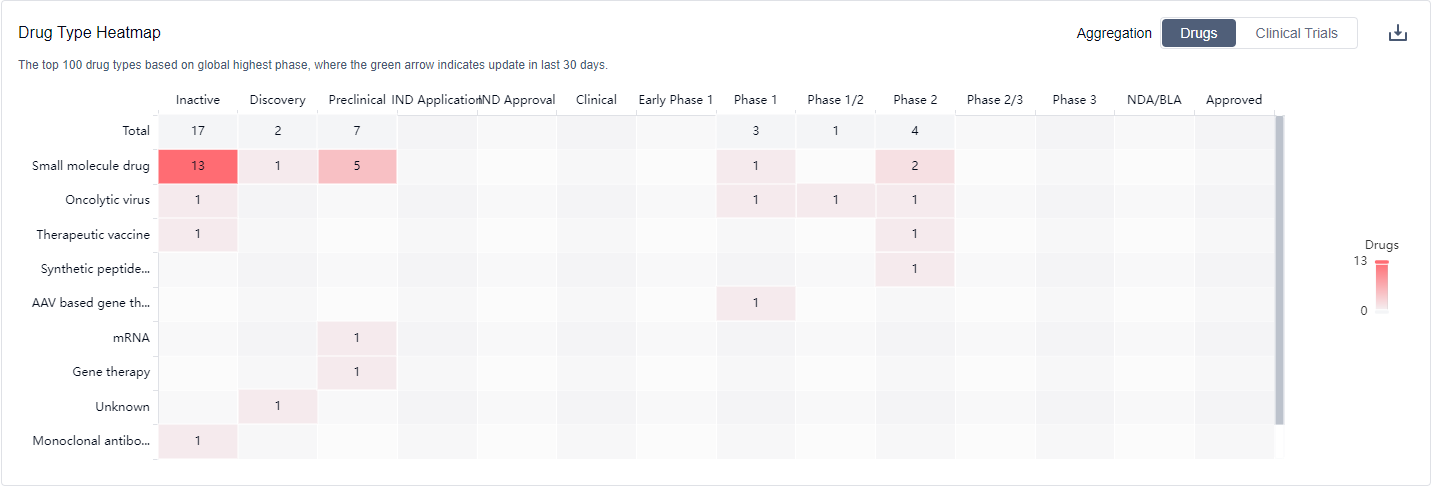
The drug types progressing most rapidly under the target Telomerase include Small molecule drugs, Oncolytic viruses, Therapeutic vaccines, Synthetic peptide vaccines, AAV based gene therapy, mRNA, Gene therapy, Unknown, Monoclonal antibodies, and Synthetic peptides. The specific research and development institutions involved in the development of biosimilars are not provided in the data.
The countries/locations developing fastest under the target Telomerase are Australia, United States, European Union, Germany, South Korea, Japan, United Kingdom, Sweden, Spain, Norway, Denmark, Belgium, Taiwan Province, China, Colombia, Israel, and Hong Kong. China has shown progress with one drug in Phase 1, but its progress is relatively limited compared to other countries/locations.
Overall, the target Telomerase presents a competitive landscape with active R&D efforts, particularly in Phase 2, and a diverse range of drug types being developed. Further research and development are needed to advance the development of drugs targeting Telomerase and gain approvals for relevant indications.
How do they work?
Telomerase inhibitors are substances or drugs that target the enzyme telomerase, which plays a role in maintaining the length of telomeres. Telomeres are protective caps at the ends of chromosomes that shorten with each cell division. Telomerase is responsible for adding repetitive DNA sequences to the telomeres, preventing them from shortening too rapidly.
In a biomedical perspective, telomerase inhibitors are being studied as potential anticancer agents. Cancer cells often have high levels of telomerase activity, allowing them to maintain their telomere length and continue dividing indefinitely. By inhibiting telomerase, these inhibitors can disrupt the ability of cancer cells to replicate and potentially lead to their death.
Telomerase inhibitors can be classified as a type of targeted therapy, as they specifically target the telomerase enzyme. They can be administered in various forms, such as small molecule drugs or biologic agents. Research is ongoing to develop effective telomerase inhibitors for the treatment of cancer, and they are being evaluated in clinical trials.
It's important to note that telomerase inhibitors are still under investigation and not yet approved for widespread clinical use. They hold promise as a potential therapeutic approach for cancer treatment, but further research is needed to determine their safety and efficacy.
List of Telomerase Inhibitors
The currently marketed Telomerase inhibitors include:
- 6-Thio-2'-Deoxyguanosine
- Sodium metaarsenite(Komipharm)
- Telomelysin
- UV1 vaccine(Ultimovacs)
- AAV-hTERT
- AGS-499
- AGS-500
- AGS-534
- RZ-002
- MAIA-2022-12
For more information, please click on the image below.
What are Telomerase inhibitors used for?
Telomerase inhibitors have been investigated for their therapeutic potential in the following indications such as cancer Vaccines, Myeloid Malignancies, Blood Cancers, Various Types of Cancer. For more information, please click on the image below to log in and search.
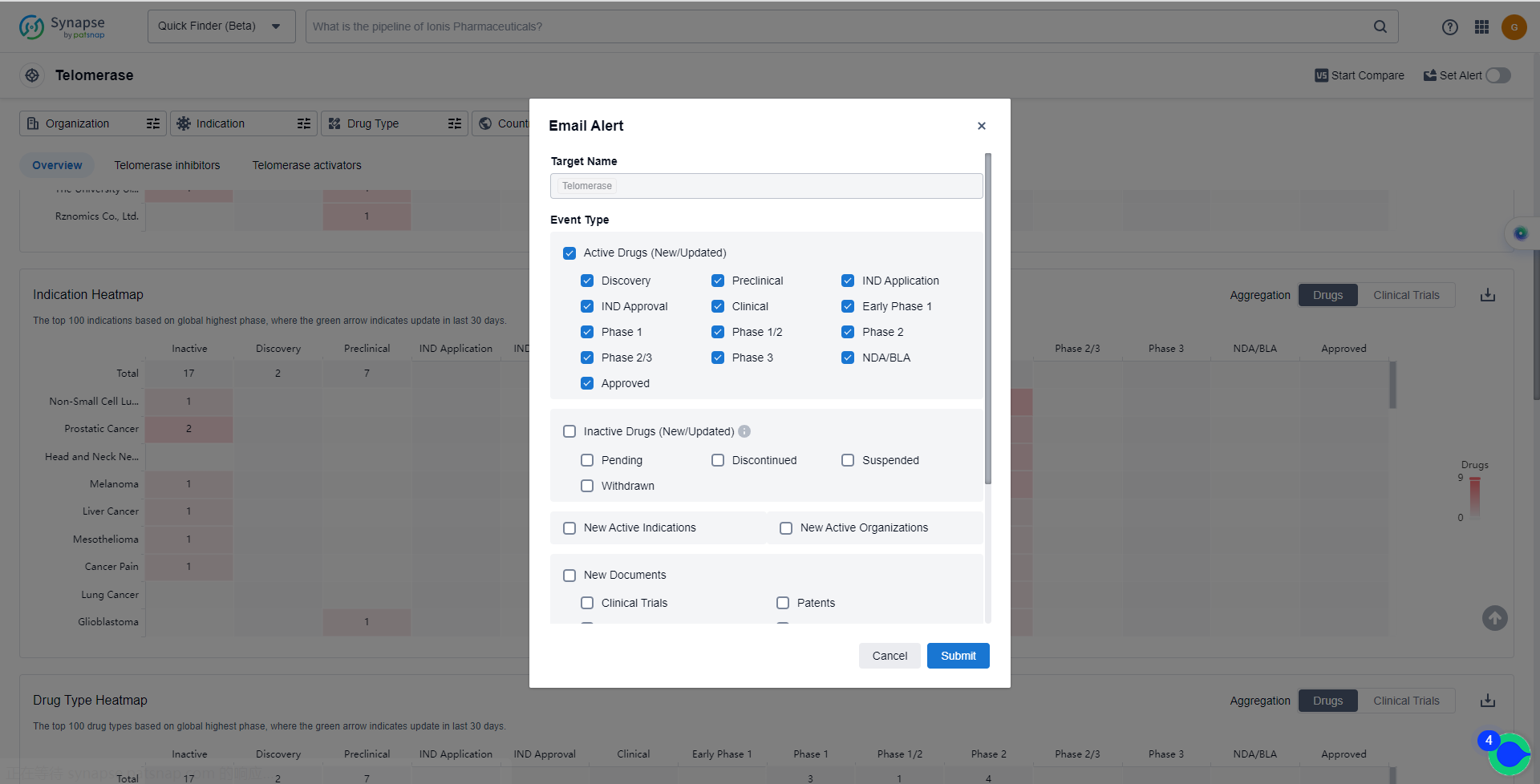
How to obtain the latest development progress of Telomerase inhibitors?
In the Synapse database, you can keep abreast of the latest research and development advances of Telomerase inhibitors anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!