Decoding Pertuzumab: A Comprehensive Study of its R&D Trends and Mechanism on Drug Target
Pertuzumab's R&D Progress
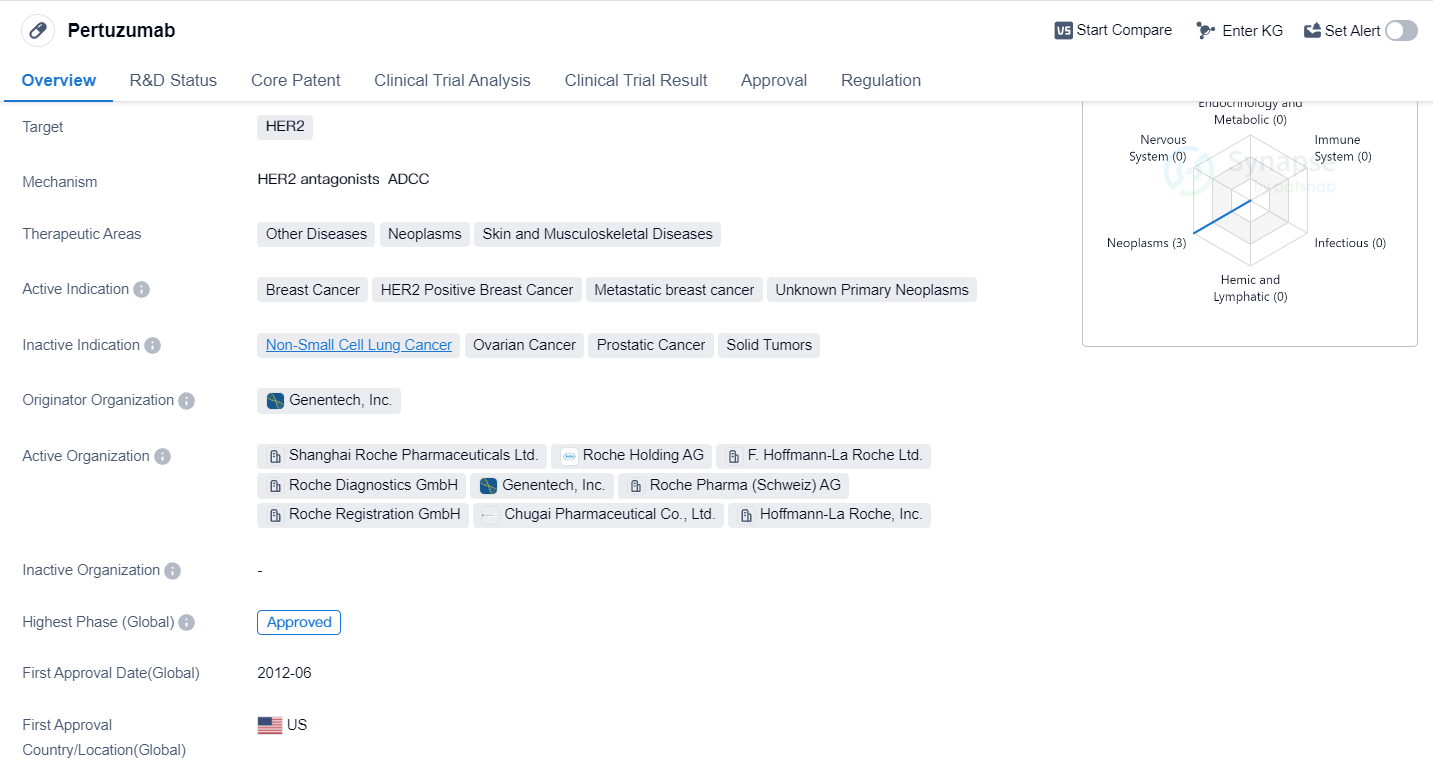
Pertuzumab is a monoclonal antibody drug that targets the HER2 protein. It is primarily used in the treatment of breast cancer, specifically HER2 positive breast cancer and metastatic breast cancer. It has also shown potential in treating other diseases, neoplasms, and skin and musculoskeletal diseases.
The drug was developed by Genentech, Inc., a leading organization in the pharmaceutical industry. Pertuzumab has received approval for use in multiple countries, including the United States, where it was first approved in June 2012. It has also been approved in China.
Pertuzumab's approval status is classified as the highest phase, indicating that it has successfully completed clinical trials and met the necessary regulatory requirements for market authorization. The drug has been granted accelerated approval. Additionally, Pertuzumab has been designated as an orphan drug, indicating that it is intended to treat rare diseases or conditions.
The specific therapeutic benefits and mechanisms of action of Pertuzumab in the treatment of breast cancer are not provided in the given information. However, as a monoclonal antibody targeting the HER2 protein, it is likely that Pertuzumab works by inhibiting the growth and spread of cancer cells that overexpress HER2.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Pertuzumab: HER2 modulators and TLR8 agonists
HER2 modulators are a class of drugs that target the human epidermal growth factor receptor 2 (HER2). HER2 is a protein that is overexpressed in certain types of cancer, particularly breast cancer. HER2 modulators work by binding to the HER2 receptor and regulating its activity. They can either inhibit the signaling pathway of HER2, preventing the growth and division of cancer cells, or they can stimulate the immune system to target and destroy HER2-positive cancer cells. These drugs are used in the treatment of HER2-positive breast cancer and have shown significant efficacy in improving patient outcomes.
TLR8 agonists are a type of drug that activate Toll-like receptor 8 (TLR8), which is a protein receptor involved in the immune response. TLR8 is primarily expressed in immune cells and plays a role in recognizing and responding to viral and bacterial infections. TLR8 agonists work by binding to TLR8 and triggering a cascade of immune responses, including the production of cytokines and activation of immune cells. This can enhance the immune system's ability to fight off infections and potentially even target cancer cells. TLR8 agonists are being investigated as potential immunotherapy agents for the treatment of infectious diseases and cancer.
In summary, HER2 modulators are drugs that target the HER2 receptor in cancer cells, while TLR8 agonists are drugs that activate the TLR8 receptor to enhance the immune response.
Drug Target R&D Trends for Pertuzumab
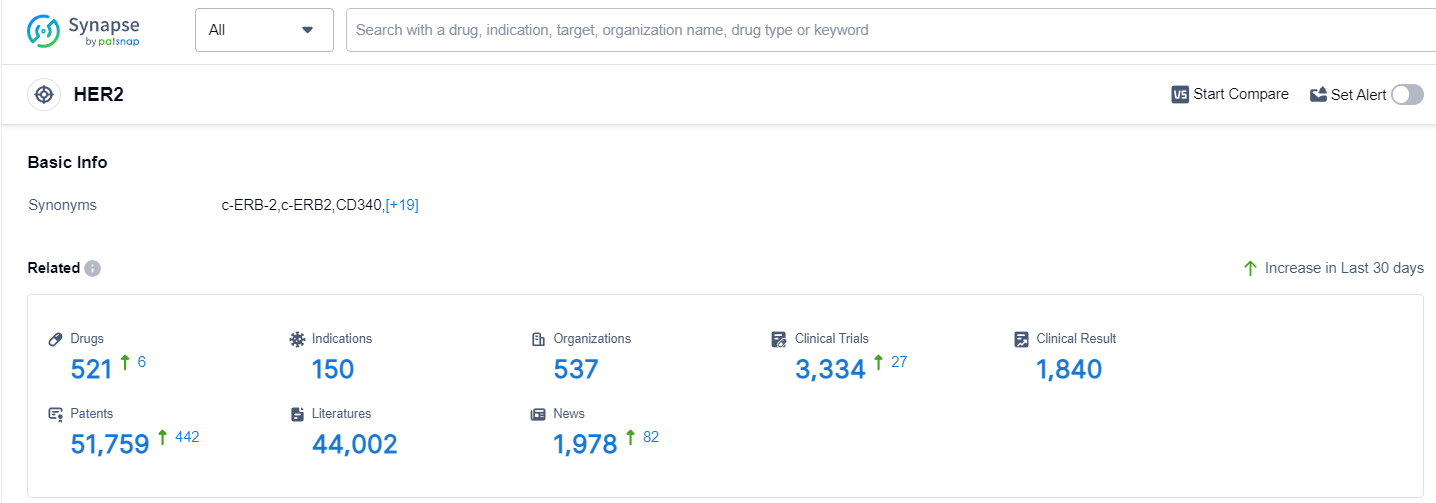
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 521 HER2 drugs worldwide, from 537 organizations, covering 150 indications, and conducting 3334 clinical trials.
The analysis of the current competitive landscape of target HER2 reveals that Roche Holding AG is the leading company in terms of R&D progress, with drugs in various stages of development. Several drugs have been approved for indications such as breast cancer, HER2 positive breast cancer, and HER2-positive gastric cancer. Monoclonal antibodies and biosimilars are the most rapidly progressing drug types, indicating intense competition. The United States and China are the countries developing fastest under the current target, with significant progress in both regions. The future development of target HER2 is expected to continue with advancements in R&D and the introduction of innovative drugs to address various indications related to HER2.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Pertuzumab is a monoclonal antibody drug developed by Genentech, Inc. It is primarily used in the treatment of breast cancer, particularly HER2 positive breast cancer and metastatic breast cancer. The drug has received approval in multiple countries, including the United States and China. Pertuzumab's approval status is the highest phase, and it has been granted accelerated approval and orphan drug designation.