Decoding TBO-Filgrastim: A Comprehensive Study of its R&D Trends
TBO-Filgrastim's R&D Progress
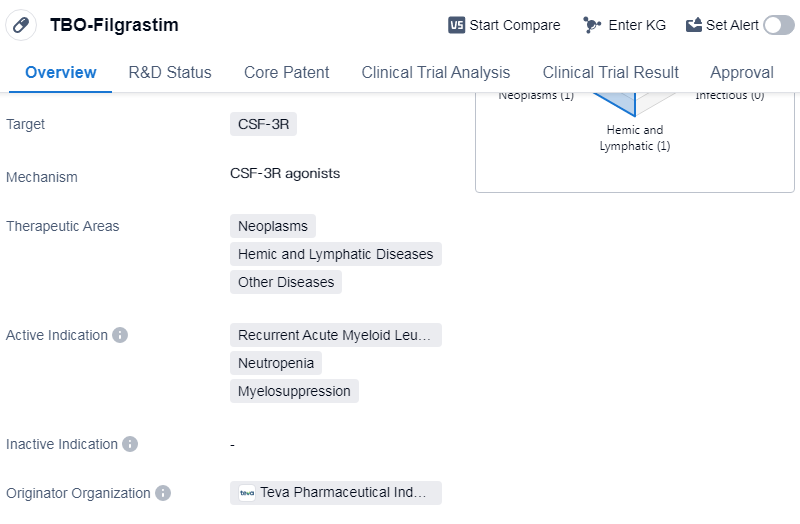
TBO-Filgrastim is a biosimilar drug that falls under the category of colony-stimulating factors in the field of biomedicine. It specifically targets CSF-3R, which is involved in the regulation of white blood cell production. The drug has been approved for use in the treatment of various conditions, including neoplasms, hemic and lymphatic diseases, and other diseases.
The active indications for TBO-Filgrastim include recurrent acute myeloid leukemia, neutropenia, and myelosuppression. These conditions are characterized by a decrease in the number of white blood cells, which can lead to an increased risk of infections and other complications. By stimulating the production of white blood cells, TBO-Filgrastim helps to restore the immune system's ability to fight off infections.
Teva Pharmaceutical Industries Ltd. is the originator organization of TBO-Filgrastim. They are a well-known pharmaceutical company that specializes in the development and production of generic and specialty medicines. TBO-Filgrastim has reached the highest phase of development, which is approval, indicating that it has successfully met the necessary regulatory requirements for market authorization.
The drug received its first approval in the European Union in September 2008. This means that it was deemed safe and effective for use in patients within the European Union. Additionally, TBO-Filgrastim has been granted orphan drug status, which is a regulatory designation given to drugs that are intended to treat rare diseases or conditions. This status provides certain incentives and benefits to the manufacturer, such as market exclusivity and financial incentives.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for TBO-Filgrastim: CSF-3R agonists
CSF-3R agonists are substances or drugs that activate the CSF-3R (colony-stimulating factor 3 receptor) in the body. CSF-3R is a receptor found on the surface of certain cells, including immune cells and bone marrow cells. When CSF-3R is activated by an agonist, it triggers a signaling pathway that promotes the growth, survival, and differentiation of these cells.
From a biomedical perspective, CSF-3R agonists can be used in the field of biomedicine to stimulate the production and function of specific immune cells, such as neutrophils. Neutrophils are a type of white blood cell that plays a crucial role in the body's defense against bacterial infections. By activating CSF-3R, CSF-3R agonists can increase the production and release of neutrophils from the bone marrow into the bloodstream, enhancing the immune response against bacterial pathogens.
CSF-3R agonists may have therapeutic potential in conditions where there is a deficiency or dysfunction of neutrophils, such as severe infections, neutropenia (low neutrophil count), or certain types of cancer treatments that suppress the immune system. By stimulating the production of neutrophils, CSF-3R agonists can help restore the immune function and improve the body's ability to fight off infections.
It's important to note that CSF-3R agonists are a specific type of drug that targets the CSF-3R receptor. They work by binding to the receptor and mimicking the action of the natural ligand (CSF-3) that normally activates the receptor. This activation of CSF-3R leads to downstream cellular responses that ultimately promote the growth and differentiation of immune cells.
Drug Target R&D Trends for TBO-Filgrastim
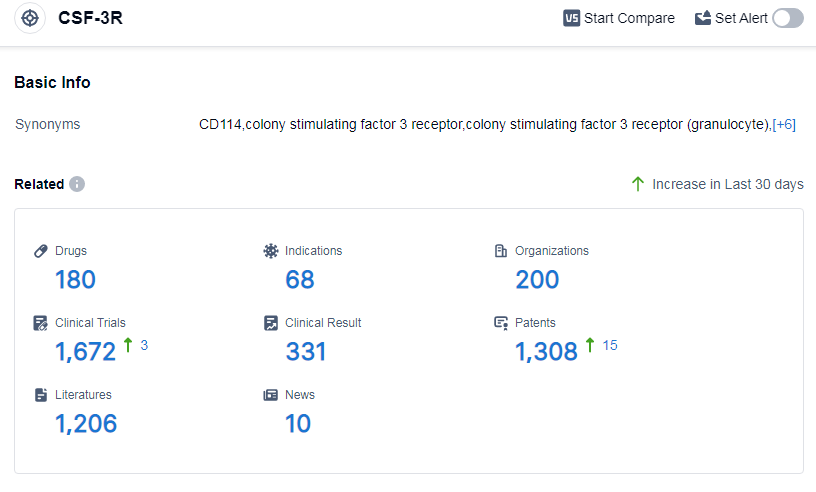
According to Patsnap Synapse, as of 15 Sep 2023, there are a total of 180 CSF-3R drugs worldwide, from 200 organizations, covering 68 indications, and conducting 1672 clinical trials.
The analysis of the current competitive landscape of target CSF-3R reveals that Teva Pharmaceutical Industries Ltd., Kirin Holdings Co., Ltd., Intas Pharmaceuticals Ltd., Emcure Pharmaceuticals Ltd., and Amgen, Inc. are the companies growing fastest under this target. The highest stage of development is the approved phase, with Teva Pharmaceutical Industries Ltd. having the highest number of drugs in this phase. The drugs under target CSF-3R have been approved for various indications, with neutropenia and febrile neutropenia having the highest number of approved drugs. The drug types progressing most rapidly are colony-stimulating factors and biosimilars, indicating intense competition around the innovative drugs. China is the country developing fastest under the current target, with the highest number of drugs in the approved phase. Other countries like India, United States, and European Union also show progress in the development of drugs for target CSF-3R. Overall, the future development of target CSF-3R looks promising with multiple companies involved in research and development.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, TBO-Filgrastim is a biosimilar drug that targets CSF-3R and is used in the treatment of neoplasms, hemic and lymphatic diseases, and other diseases. The drug was developed by Teva Pharmaceutical Industries Ltd.It has been approved for use in the European Union since 2008 and has orphan drug status.