An In-depth Analysis of Tolcapone's R&D Progress and Mechanism of Action on Drug Target
Tolcapone's R&D Progress
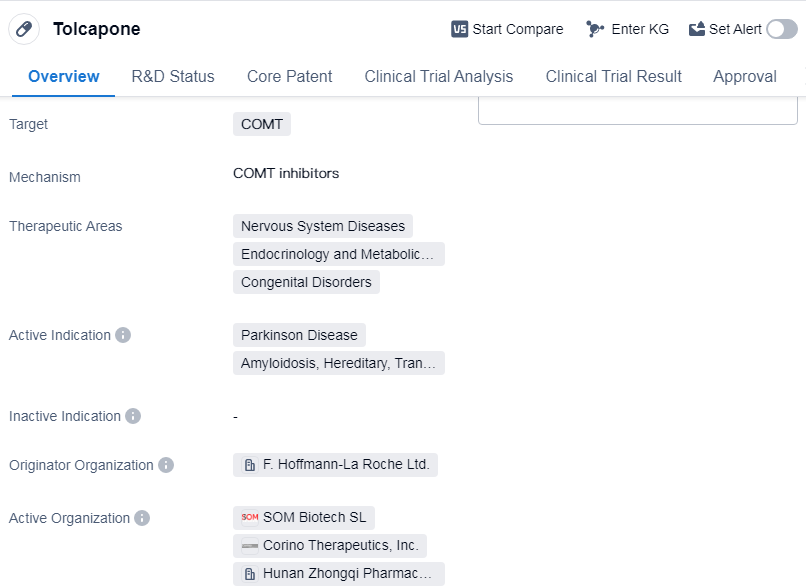
Tolcapone is a small molecule drug that targets the enzyme catechol-O-methyltransferase (COMT). It is primarily used in the treatment of various nervous system diseases, endocrinology and metabolic diseases, as well as congenital disorders. The drug has been approved for the treatment of Parkinson's disease, amyloidosis (specifically hereditary transthyretin-related amyloidosis).
Tolcapone was developed by F. Hoffmann-La Roche Ltd., a renowned pharmaceutical company. It received its first approval in the European Union in August 1997, making it available for patients in that region. The drug has also obtained approval in China, indicating its global reach and potential impact on patients worldwide.
One notable aspect of Tolcapone is its regulatory status as an orphan drug. Orphan drugs are medications developed to treat rare diseases or conditions that affect a small number of individuals. This designation provides certain incentives to pharmaceutical companies, such as extended market exclusivity and financial support, to encourage the development of treatments for these rare conditions.
The approval of Tolcapone in both the European Union and China suggests that it has undergone rigorous testing and evaluation to ensure its safety and efficacy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Tolcapone: COMT inhibitors
COMT inhibitors are a type of medication used in the field of biomedicine to treat certain medical conditions. COMT stands for catechol-O-methyltransferase, which is an enzyme involved in the breakdown of certain neurotransmitters in the brain, such as dopamine, epinephrine, and norepinephrine. By inhibiting the activity of COMT, these inhibitors help to increase the levels of these neurotransmitters in the brain.
In a biomedical perspective, COMT inhibitors are primarily used in the treatment of Parkinson's disease. Parkinson's disease is a neurodegenerative disorder characterized by a deficiency of dopamine in the brain. By inhibiting COMT, these medications prolong the effects of dopamine, thereby helping to alleviate the motor symptoms associated with Parkinson's disease, such as tremors, stiffness, and difficulty with movement.
COMT inhibitors can also be used in combination with other medications, such as levodopa, to enhance the therapeutic effects and reduce the side effects of levodopa. Levodopa is a precursor of dopamine that is commonly used in the treatment of Parkinson's disease. However, it is rapidly broken down by COMT, leading to fluctuations in dopamine levels and the development of motor complications. By inhibiting COMT, these inhibitors help to maintain more stable dopamine levels and improve the overall management of Parkinson's disease.
It is important to note that COMT inhibitors may have potential side effects, including nausea, vomiting, diarrhea, and hallucinations. Therefore, their use should be closely monitored by healthcare professionals, and the benefits and risks should be carefully considered for each individual patient.
Drug Target R&D Trends for Tolcapone
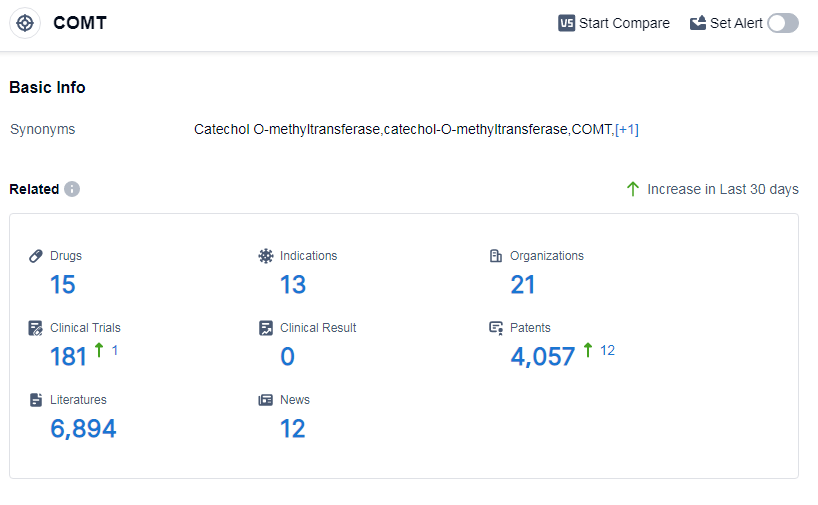
According to Patsnap Synapse, as of 15 Sep 2023, there are a total of 15 COMT drugs worldwide, from 21 organizations, covering 13 indications, and conducting 181 clinical trials.
The analysis of the current competitive landscape of target COMT reveals that Orion Pharma Ltd., Orion Oyj, Novartis AG, BIAL-Portela & Cia SA, and Neurocrine Biosciences, Inc. are some of the companies growing fastest under this target. The highest stage of development is the approved phase, with a significant number of drugs. These drugs have been approved for indications such as Parkinson's disease, muscle spasticity, and other neurological disorders. Small molecule drugs are progressing rapidly, indicating intense competition in the market. The European Union, United States, China, and Japan are the countries/locations with the highest development under this target, with China showing significant progress. Overall, the target COMT presents a competitive landscape with multiple companies and drug types, indicating a promising future for the development of drugs targeting this enzyme.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Tolcapone is a small molecule drug developed by F. Hoffmann-La Roche Ltd. It targets the enzyme COMT and has been approved for the treatment of Parkinson's disease, amyloidosis (specifically hereditary transthyretin-related amyloidosis), and other related conditions. Its first approval was granted in the European Union in 1997, and it has also received approval in China. The drug's orphan drug status highlights its potential to address unmet medical needs in rare diseases.