Triclabendazole: Detailed Review of its Transformative R&D Success
Triclabendazole's R&D Progress
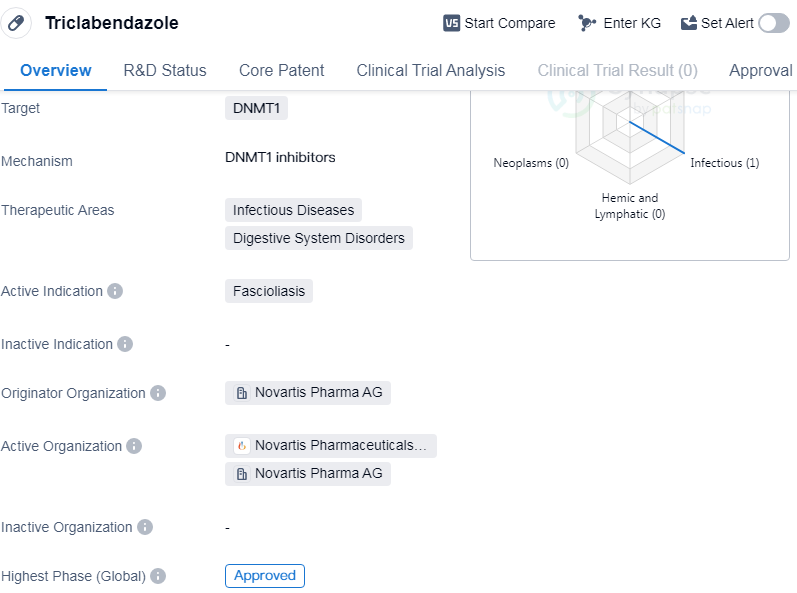
Triclabendazole is a small molecule drug that primarily targets DNMT1. It is used in the treatment of Fascioliasis, a parasitic infection that affects the liver and bile ducts. The drug was first approved in 1989 in Iran and is currently approved in several countries globally.
Triclabendazole is classified as a small molecule drug, which means it is a low molecular weight compound that can easily penetrate cell membranes. This characteristic makes it suitable for oral administration, allowing for convenient and effective treatment of Fascioliasis.
The primary target of Triclabendazole is DNMT1, which stands for DNA methyltransferase 1. DNMT1 is an enzyme involved in the process of DNA methylation, which plays a crucial role in gene regulation. By targeting DNMT1, Triclabendazole disrupts the methylation process, leading to the inhibition of parasite growth and replication.
Fascioliasis is an infectious disease that affects the liver and bile ducts. It is caused by the parasitic flatworms Fasciola hepatica and Fasciola gigantica. These parasites are commonly found in livestock, and humans can become infected by consuming contaminated water or plants. Triclabendazole has shown efficacy in treating Fascioliasis by killing the parasites and reducing the associated symptoms.
Triclabendazole was initially developed by Novartis Pharma AG, a leading pharmaceutical company. It received its first approval in Iran in 1989 and has since been approved in several other countries globally. The drug has been granted fast-track and orphan drug designations, indicating its potential to address unmet medical needs and expedite the regulatory process.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Triclabendazole: DNMT1 inhibitors
DNMT1 inhibitors are a type of drugs that target and inhibit the activity of the enzyme DNA methyltransferase 1 (DNMT1). DNMT1 is responsible for adding methyl groups to DNA molecules, a process known as DNA methylation. DNA methylation plays a crucial role in gene regulation and silencing, and abnormal DNA methylation patterns have been associated with various diseases, including cancer.
By inhibiting DNMT1, DNMT1 inhibitors can alter the pattern of DNA methylation, leading to changes in gene expression.
From a biomedical perspective, DNMT1 inhibitors hold promise in epigenetic therapy, which aims to modify gene expression patterns without altering the underlying DNA sequence.
Drug Target R&D Trends for Triclabendazole
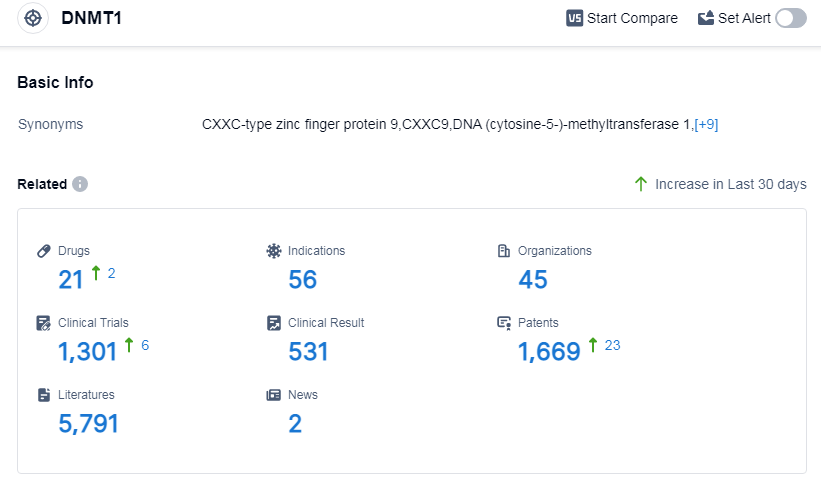
According to Patsnap Synapse, as of 13 Sep 2023, there are a total of 21 DNMT1 drugs worldwide, from 45 organizations, covering 56 indications, and conducting 1301 clinical trials.
The analysis of the current competitive landscape of target DNMT1 reveals that Otsuka Holdings Co., Ltd., Eisai Co., Ltd., Bristol Myers Squibb Co., Johnson & Johnson, and other companies are leading in terms of R&D progress. Triclabendazole has gained the global approval, with drugs approved for indications such as myelodysplastic syndromes, chronic myelomonocytic leukemia, acute myeloid leukemia, and others. Small molecule drugs are progressing rapidly, indicating intense competition in the market. The United States, China, European Union, and Japan are the countries/locations with the fastest development, with China showing significant progress. Overall, the target DNMT1 has a competitive landscape with a focus on small molecule drugs and a global presence in terms of R&D efforts.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Triclabendazole is a small molecule drug that targets DNMT1 and is used in the treatment of Fascioliasis. It was first approved in Iran in 1989 and has since gained approval in multiple countries. The drug's mechanism of action involves disrupting DNA methylation, leading to the inhibition of parasite growth. Triclabendazole has been recognized for its potential in addressing unmet medical needs and has received fast-track and orphan drug designations.