Deep Scientific Insights on Vandetanib's R&D Progress
Vandetanib's R&D Progress
Vandetanib is a small molecule drug that belongs to the field of biomedicine. It targets multiple proteins including BRK, EGFR, Eph, RET, SRC family, Tie-2, and VEGFR. The drug has shown potential therapeutic benefits in the treatment of neoplasms, respiratory diseases, and endocrinology and metabolic diseases.
Vandetanib is specifically indicated for the treatment of RET Mutation-Positive Medullary Thyroid Cancer, Thyroid Cancer, Thyroid Cancer (Medullary), and Small Cell Lung Cancer. It was developed by AstraZeneca Pharmaceutical Co., Ltd., a renowned pharmaceutical organization.
The drug has reached the highest phase of development, which is approved globally. It received its first approval in the United States in April 2011. Vandetanib has also undergone Phase 3 clinical trials in China, indicating its progress towards potential approval in the Chinese market.
One notable aspect of Vandetanib is its regulatory status as an orphan drug. Orphan drugs are medications developed to treat rare diseases or conditions that affect a small number of patients. This designation provides certain incentives to pharmaceutical companies, such as market exclusivity and financial benefits, to encourage the development of drugs for rare diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Vandetanib: BRK inhibitors & EGFR antagonists & Eph inhibitors & RET inhibitors & SRC inhibitors & Tie-2 antagonists & VEGFR antagonists
These are different types of inhibitors that target specific proteins or receptors involved in various biological processes.
1. BRK inhibitors: These are inhibitors that specifically target the protein BRK (Breast tumor kinase), which is involved in cell signaling pathways. Inhibition of BRK can potentially be used to treat certain types of cancer.
2. EGFR antagonists: EGFR (Epidermal Growth Factor Receptor) antagonists are drugs that inhibit the activity of the EGFR protein. EGFR is involved in cell growth and division, and its overactivation is associated with the development and progression of certain cancers. Antagonists of EGFR can be used to block its activity and potentially slow down the growth of cancer cells.
3. Eph inhibitors: Eph inhibitors target the Eph receptors, a family of proteins involved in cell communication and tissue development. These inhibitors can interfere with the signaling pathways mediated by Eph receptors and have potential applications in cancer therapy and tissue regeneration.
4. RET inhibitors: RET (REarranged during Transfection) inhibitors are drugs that target the RET protein, which is a receptor tyrosine kinase involved in cell growth and differentiation. Inhibition of RET can be beneficial in the treatment of certain types of cancer, particularly those driven by abnormal RET activity.
5. SRC inhibitors: SRC (Steroid Receptor Coactivator) inhibitors are compounds that specifically inhibit the activity of the SRC protein. SRC is involved in cellular processes such as cell growth, migration, and invasion. Inhibition of SRC can be explored as a therapeutic strategy for cancer treatment.
6. Tie-2 antagonists: Tie-2 antagonists target the Tie-2 receptor, which is involved in angiogenesis (the formation of new blood vessels) and vascular stability. By blocking the Tie-2 receptor, these antagonists can potentially inhibit angiogenesis and have applications in cancer therapy and other diseases characterized by abnormal blood vessel formation.
7. VEGFR antagonists: VEGFR (Vascular Endothelial Growth Factor Receptor) antagonists are drugs that inhibit the activity of VEGFR, a receptor involved in angiogenesis. By blocking VEGFR, these antagonists can interfere with the formation of new blood vessels, which is crucial for tumor growth and metastasis. VEGFR antagonists are used in the treatment of certain types of cancer and other diseases involving abnormal angiogenesis.
Drug Target R&D Trends for Vandetanib
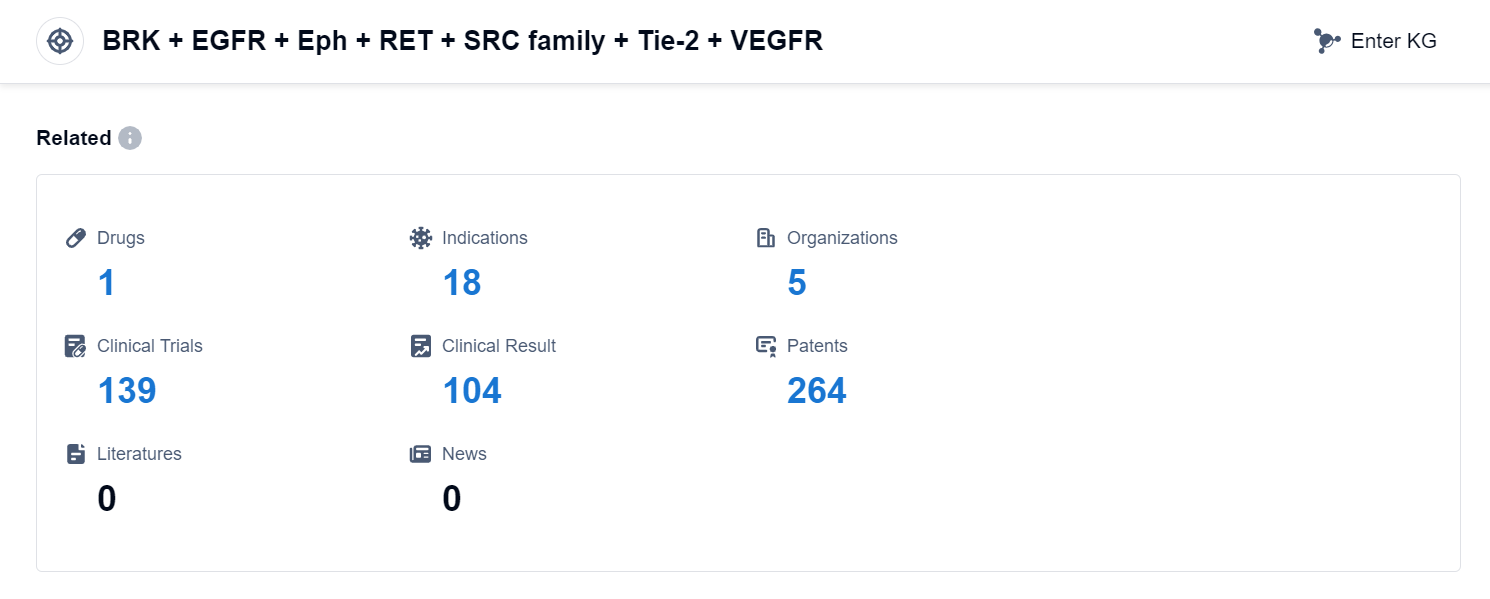
According to Patsnap Synapse, as of 10 Sep 2023, there are a total of 1 BRK + EGFR + Eph + RET + SRC family + Tie-2 + VEGFR drugs worldwide, from 5 organizations, covering 18 indications, and conducting 139 clinical trials.
The analysis of the target BRK + EGFR + Eph + RET + SRC family + Tie-2 + VEGFR reveals a competitive landscape with multiple companies involved in the research and development of drugs. AstraZeneca PLC and Sanofi are the companies with the highest phase of development, indicating their significant progress in R&D. The approved indications primarily focus on treating thyroid cancer and small cell lung cancer, while other indications are still under development. Small molecule drugs are the most rapidly progressing drug type, with potential competition from biosimilars. Various countries/locations, including China and India, are actively developing drugs targeting this target, indicating a global interest in this area of research. Overall, the target BRK + EGFR + Eph + RET + SRC family + Tie-2 + VEGFR shows promise for future development in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Vandetanib is a small molecule drug developed by AstraZeneca Pharmaceutical Co., Ltd. It targets multiple proteins and has shown potential therapeutic benefits in the treatment of various diseases, including neoplasms, respiratory diseases, and endocrinology and metabolic diseases. The drug is specifically indicated for RET Mutation-Positive Medullary Thyroid Cancer, Thyroid Cancer, Thyroid Cancer (Medullary), and Small Cell Lung Cancer. It has received approval globally and was first approved in the United States in 2011. Vandetanib has also undergone Phase 3 clinical trials in China and holds the regulatory status of an orphan drug.