Fam-trastuzumab deruxtecan-NXKI: Detailed Review of its Transformative R&D Success
Fam-trastuzumab deruxtecan-NXKI's R&D Progress
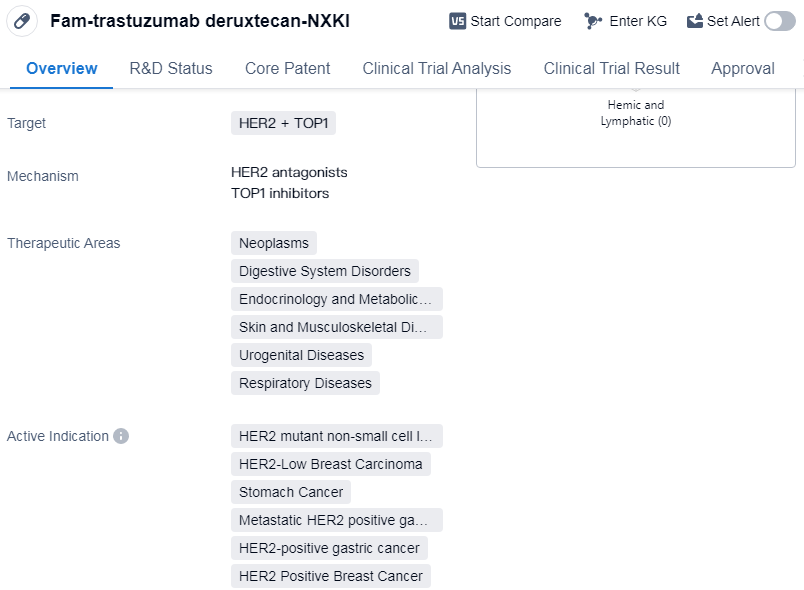
Fam-trastuzumab deruxtecan-NXKI is a drug classified as a monoclonal antibody and an antibody drug conjugate (ADC). It targets human epidermal growth factor receptor 2 (HER2) and topoisomerase I(TOP1), making it suitable for the treatment of various neoplasms, digestive system disorders, endocrinology and metabolic diseases, skin and musculoskeletal diseases, urogenital diseases, and respiratory diseases.
The drug has been approved for several indications, including HER2 mutant non-small cell lung cancer, HER2-low breast carcinoma, stomach cancer, metastatic HER2 positive gastroesophageal junction cancer, HER2-positive gastric cancer, etc.
The drug was developed by Daiichi Sankyo Co., Ltd., and it has received approvals in global markets. The highest phase of development for this drug is approved, indicating that it has successfully completed clinical trials and met the necessary regulatory requirements for market authorization.
Fam-trastuzumab deruxtecan-NXKI received its first approval in the United States in December 2019. The drug has undergone regulatory processes such as priority review, SAKIGAKE designation (a fast-track system in Japan), accelerated approval, fast-track designation, orphan drug designation, and breakthrough therapy designation. These regulatory designations highlight the potential significance and urgency of the drug in addressing unmet medical needs.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Fam-trastuzumab deruxtecan-NXKI: HER2 Antagonists and TOP1 Inhibitors
HER2 antagonists are a class of drugs that specifically target HER2. HER2 is a protein that is overexpressed in certain types of cancer, such as breast cancer. HER2 antagonists work by binding to the HER2 receptor and blocking its activity, thereby inhibiting the growth and spread of cancer cells that depend on HER2 signaling for their survival. These drugs are commonly used in the treatment of HER2-positive breast cancer.
TOP1 inhibitors, on the other hand, are a type of drug that targets the enzyme TOP1. TOP1 is involved in DNA replication and repair processes within cells. Inhibiting TOP1 can prevent the proper functioning of DNA and ultimately lead to cell death. TOP1 inhibitors are primarily used in cancer treatment as they can selectively target and kill cancer cells that rely heavily on DNA replication. These inhibitors are particularly effective against certain types of solid tumors, such as colorectal cancer.
In summary, HER2 antagonists are drugs that target the HER2 receptor in cancer cells, while TOP1 inhibitors target the enzyme TOP1 involved in DNA replication and repair. Both types of drugs play a crucial role in the treatment of specific types of cancer.
Drug Target R&D Trends for Fam-trastuzumab deruxtecan-NXKI
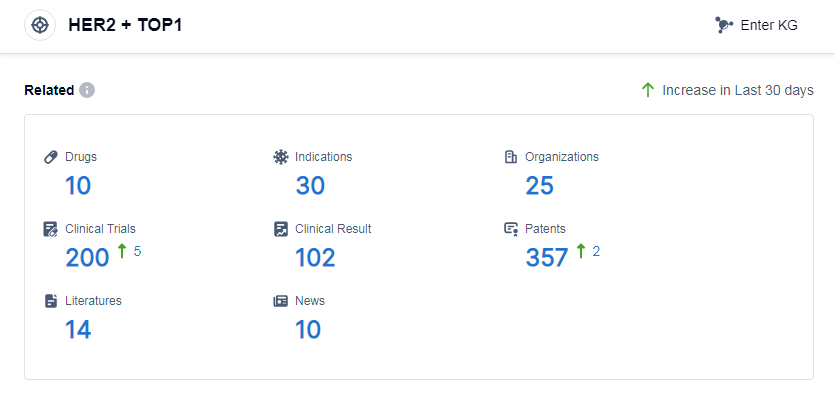
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 10 HER2 and TOP1 drugs worldwide, from 25 organizations, covering 30 indications, and conducting 200 clinical trials.
The analysis of the target HER2 and TOP1 reveals a competitive landscape with multiple companies, indications, drug types, and countries/locations involved in the development of drugs. Daiichi Sankyo Co., Ltd. and AstraZeneca PLC are the companies with the highest stage of development on this target. The approved indications cover a range of cancers, including breast, gastric, stomach, lung, and gastroesophageal junction cancers. The most rapidly progressing drug types includeADC, monoclonal antibody, and bispecific antibody. China, the United States, and other countries have shown significant progress in the development of drugs targeting HER2 and TOP1. Overall, the target HER2 and TOP1 presents a promising area for further research and development in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Fam-trastuzumab deruxtecan-NXKI is a monoclonal antibody and ADC that targets HER2 and TOP1. It has been approved for various indications in the field of biomedicine, particularly in the treatment of different types of cancers. Developed by Daiichi Sankyo Co., Ltd., the drug has successfully completed clinical trials and received approvals in global markets. Its regulatory designations further emphasize its potential as a breakthrough therapy for patients with unmet medical needs.