Deep Scientific Insights on Idarucizumab's R&D Progress
Idarucizumab's R&D Progress
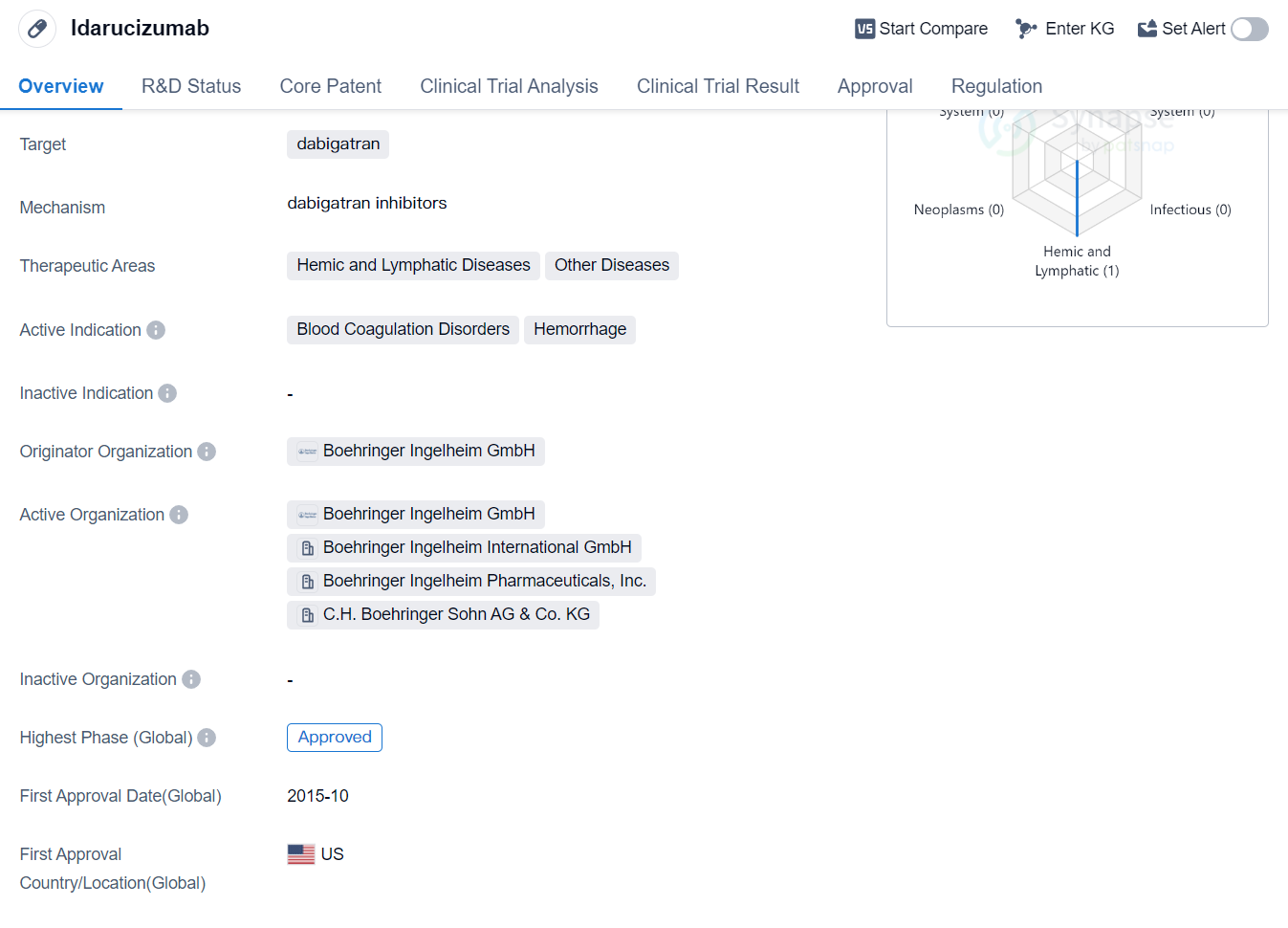
Idarucizumab is a drug that falls under the category of Fab fragment, Monoclonal antibody. It is primarily designed to target dabigatran, a medication used to prevent blood clots. The therapeutic areas in which Idarucizumab is used include Hemic and Lymphatic Diseases, as well as Other Diseases. The drug is specifically indicated for the treatment of Blood Coagulation Disorders and Hemorrhage.
The originator organization responsible for the development and production of Idarucizumab is Boehringer Ingelheim GmbH, a pharmaceutical company known for its expertise in the field. The drug has successfully completed the highest phase of clinical trials and has received approval globally.
Idarucizumab was first approved for use in the United States in October 2015. It is important to note that the drug has been classified as an Orphan Drug, which means it is intended to treat rare diseases or conditions that affect a small number of patients.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Idarucizumab: dabigatran inhibitor
Dabigatran inhibitors are a type of medication that belong to the class of direct oral anticoagulants (DOACs). Dabigatran is the active ingredient in these inhibitors and it works by inhibiting the action of thrombin, which is an enzyme involved in blood clotting. By blocking thrombin, dabigatran inhibitors help prevent the formation of blood clots in individuals who are at risk of conditions such as stroke or deep vein thrombosis.
These inhibitors are commonly used in the treatment and prevention of various conditions related to abnormal blood clotting, including atrial fibrillation, deep vein thrombosis, and pulmonary embolism. They are considered an alternative to traditional anticoagulants like warfarin, as they have a more predictable effect and do not require frequent monitoring of blood levels.
It's important to note that dabigatran inhibitors are prescription drugs and should only be used under the supervision of a healthcare professional. They may have potential side effects and can interact with other medications, so it's crucial to follow the prescribed dosage and consult with a healthcare provider for proper management.
Drug Target R&D Trends for Idarucizumab
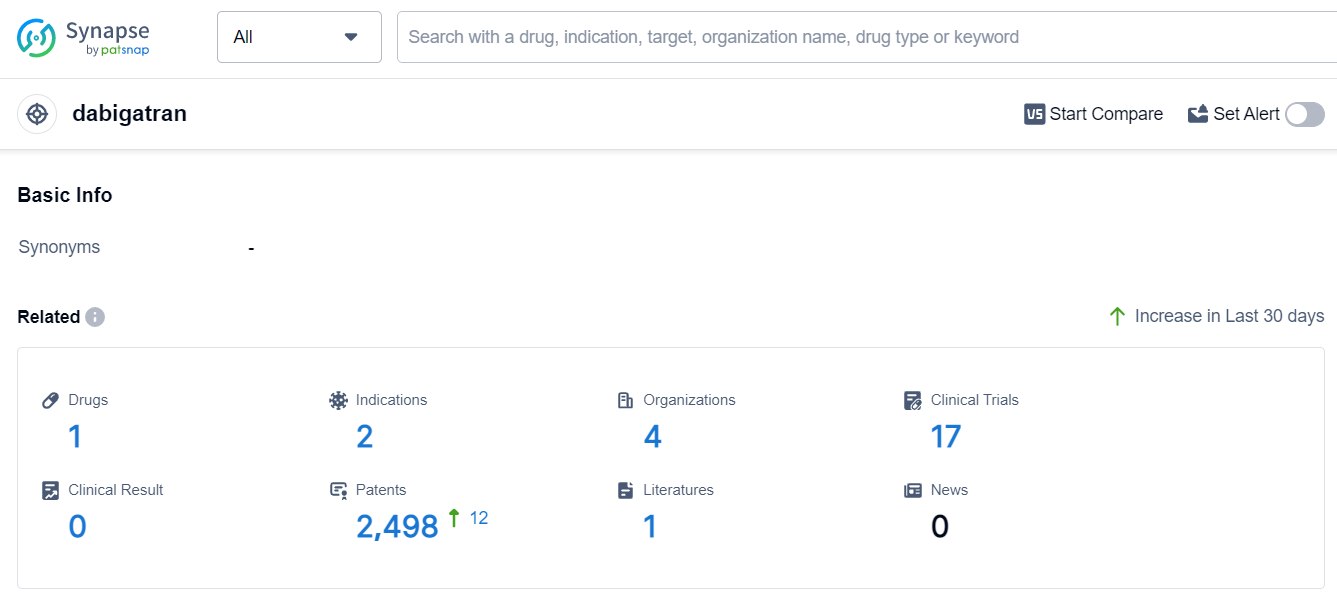
The analysis of the current competitive landscape of target dabigatran reveals that C.H. Boehringer Sohn AG & Co. KG is the leading company in terms of development stages. The indications of hemorrhage and blood coagulation disorders have been approved for drugs under this target. Fab fragment and monoclonal antibody are the drug types progressing rapidly, indicating intense competition. Various countries, including China, are actively developing dabigatran, highlighting its global significance. The future development of dabigatran is promising, with ongoing research and development efforts focused on different stages, indications, and drug types.
According to Patsnap Synapse, as of 9 Sep 2023, there are a total of 1 dabigatran drugs worldwide, from 4 organizations, covering 2 indications, and conducting 17 clinical trials.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Idarucizumab is a Fab fragment, Monoclonal antibody drug developed by Boehringer Ingelheim GmbH. It specifically targets dabigatran and is used to treat Blood Coagulation Disorders and Hemorrhage. The drug has received approval in both the United States and China and is classified as an Orphan Drug. Its first approval date was in October 2015.