Fezolinetant: Detailed Review of its Transformative R&D Success
Fezolinetant's R&D Progress
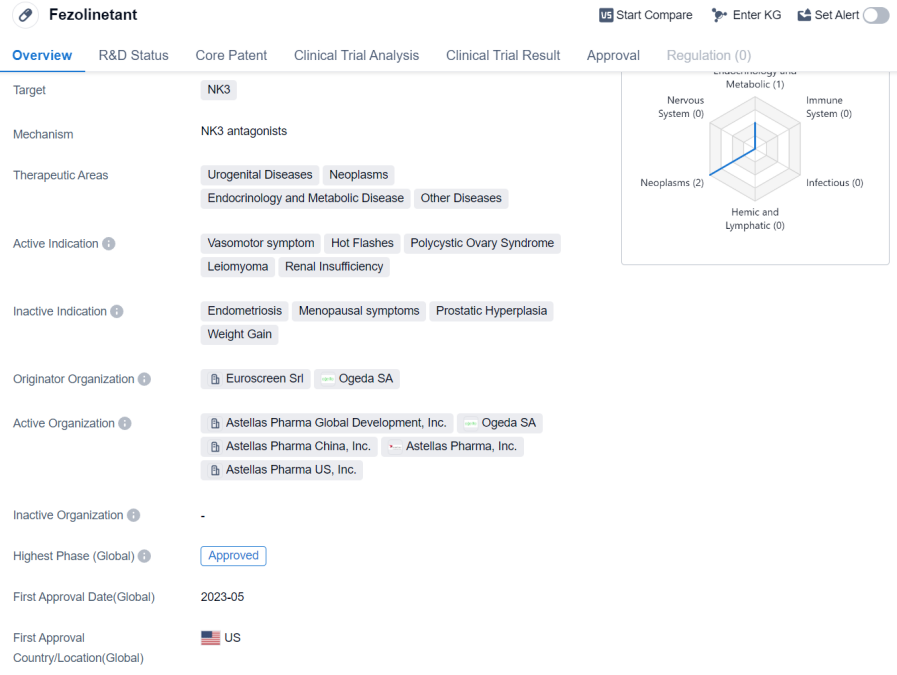
Fezolinetant is a small molecule drug that targets the NK3 receptor. It is primarily used in the treatment of urogenital diseases, neoplasms, endocrinology and metabolic diseases, and other diseases. The drug has shown efficacy in treating vasomotor symptoms, hot flashes, polycystic ovary syndrome, leiomyoma, and renal insufficiency.
Fezolinetant was developed by Euroscreen Srl and Ogeda SA. It has reached the highest phase of development, which is approved globally. In China, it is currently in phase 3 of development.
The first approval of Fezolinetant is expected to take place in May 2023, with the United States being the first country/location to grant approval.
Fezolinetant's mechanism of action involves targeting the NK3 receptor. This receptor is involved in regulating the release of gonadotropin-releasing hormone (GnRH), which plays a crucial role in the regulation of reproductive function. By targeting the NK3 receptor, Fezolinetant aims to modulate the release of GnRH and subsequently impact the hormonal balance in the body.
The drug's therapeutic potential lies in its ability to alleviate symptoms associated with urogenital diseases, neoplasms, endocrinology and metabolic diseases, and other conditions. Vasomotor symptoms, such as hot flashes, are commonly experienced by menopausal women and can significantly impact their quality of life. Fezolinetant has shown promise in reducing the frequency and severity of hot flashes, providing relief to affected individuals.
Additionally, Fezolinetant may have potential applications in the treatment of polycystic ovary syndrome, leiomyoma, and renal insufficiency. These conditions are characterized by hormonal imbalances and can lead to various complications. Fezolinetant's ability to modulate hormonal activity may offer therapeutic benefits in managing these conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Fezolinetant: NK3 antagonist
NK3 antagonists are a class of drugs that target and block the activity of the neurokinin-3 (NK3) receptor. The NK3 receptor is a type of receptor found in the central nervous system and is involved in regulating various physiological processes. By blocking the NK3 receptor, NK3 antagonists can modulate the activity of neuropeptides that bind to this receptor, such as neurokinin B.
From a biomedical perspective, NK3 antagonists have been studied for their potential therapeutic applications in various conditions. For example, they have shown promise in the treatment of psychiatric disorders like depression and anxiety. By blocking the NK3 receptor, these drugs may help regulate the release of neuropeptides involved in mood regulation.
Additionally, NK3 antagonists have been investigated for their potential role in managing symptoms associated with menopause, such as hot flashes. Neurokinin B, which acts on the NK3 receptor, is thought to play a role in the regulation of body temperature, and blocking its activity with NK3 antagonists may help alleviate hot flashes.
It's important to note that while NK3 antagonists have shown potential in preclinical and early clinical studies, further research is needed to fully understand their safety and efficacy profiles.
Drug Target R&D Trends for Fezolinetant
Based on the analysis of the provided data, the current competitive landscape of target NK3 shows that companies like Astellas Pharma, Inc., Epics Therapeutics SA, and Bayer AG are growing fastest and have made significant R&D progress. Drugs under the target NK3 have been approved for indications such as Vasomotor symptom, Hot Flashes, Polycystic Ovary Syndrome, and Menopausal symptoms. Small molecule drugs are progressing rapidly, indicating potential innovation in this area. The countries/locations developing fastest include the United States, European Union, United Kingdom, and Germany. While there is some progress in China, it is relatively limited compared to other countries/locations. Overall, the future development of target NK3 holds promise with ongoing R&D efforts and potential advancements in treatment options for various indications.
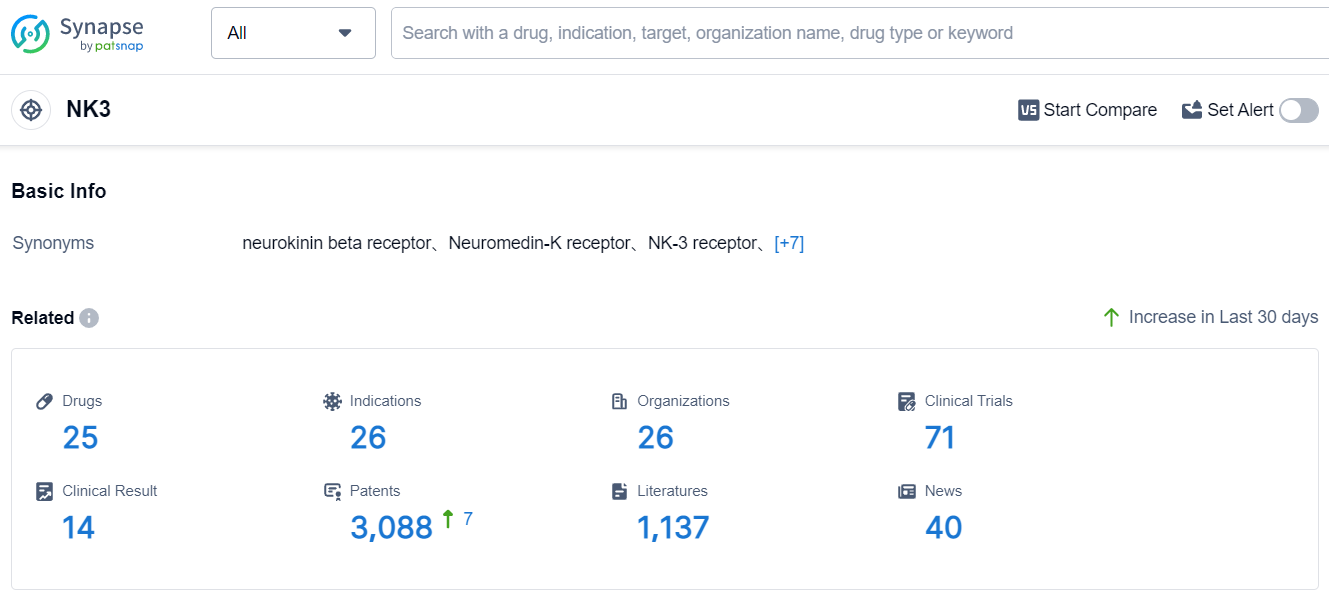
According to Patsnap Synapse, as of 6 Sep 2023, there are a total of 25 NK3 drugs worldwide, from 26 organizations, covering 26 indications, and conducting 71 clinical trials.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Fezolinetant is a small molecule drug that targets the NK3 receptor. It is being developed by Euroscreen Srl and Ogeda SA and has reached the highest phase of development, which is approved globally. The drug shows potential in treating urogenital diseases, neoplasms, endocrinology and metabolic diseases, and other conditions. Its first approval is expected in May 2023 in the United States.