Deep Scientific Insights on Thiothixene's R&D Progress, Mechanism of Action, and Drug Target
Thiothixene's R&D Progress
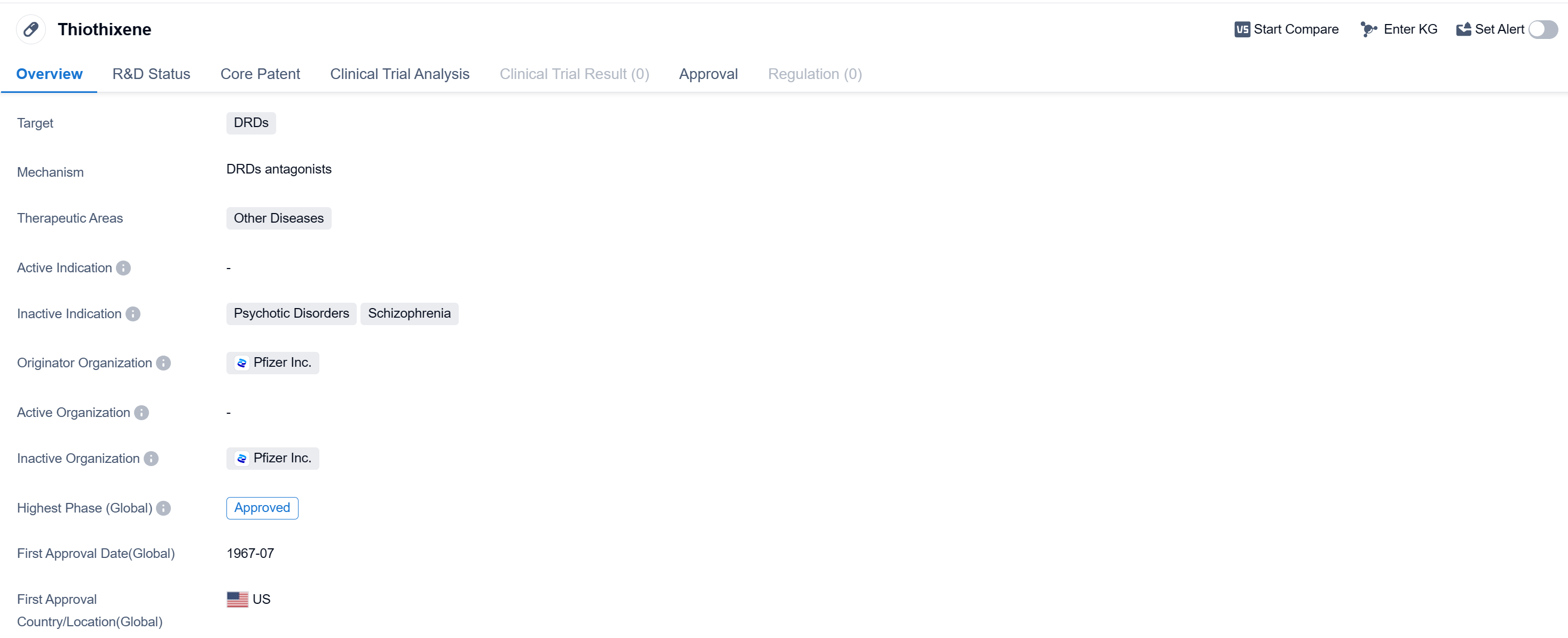
Thiothixene is a small molecule drug that falls under the therapeutic area of Other Diseases. It primarily targets DRDs (Dopamine Receptors). The drug was first approved in the United States in July 1967 and is currently in the highest phase of development, which is Approved.
Thiothixene is developed by Pfizer Inc., a renowned pharmaceutical company. As a small molecule drug, it is composed of a relatively low molecular weight and is typically orally administered. Small molecule drugs are known for their ability to penetrate cell membranes and interact with specific targets within the body.
The specific therapeutic areas for Thiothixene are categorized as Other Diseases. This indicates that the drug is primarily used to treat conditions that do not fall under more specific therapeutic categories. However, without further information, it is difficult to determine the exact diseases or conditions that Thiothixene targets within this category.
The drug's target, DRDs, refers to Dopamine Receptors. Dopamine is a neurotransmitter that plays a crucial role in various physiological processes, including movement, motivation, and reward. By targeting DRDs, Thiothixene may modulate dopamine signaling and potentially alleviate symptoms associated with diseases or conditions related to dopamine dysregulation.
Thiothixene received its first approval in the United States in July 1967. The approval in the United States indicates that Thiothixene has met the necessary requirements set by the regulatory authorities in that country.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Thiothixene: DRDs antagonists
DRDs antagonists refers to dopamine receptor antagonists. Dopamine receptors are a type of G protein-coupled receptors found in the central nervous system. They play a crucial role in various physiological processes, including motor control, reward, mood regulation, and cognition. DRDs antagonists are drugs that bind to dopamine receptors and block their activity, thereby inhibiting the effects of dopamine.
From a biomedical perspective, DRDs antagonists are commonly used in the treatment of psychiatric disorders such as schizophrenia, bipolar disorder, and certain types of depression. By blocking dopamine receptors, these antagonists help to regulate the excessive dopamine signaling that is often associated with these conditions. This can help alleviate symptoms such as hallucinations, delusions, and mood disturbances.
It's important to note that dopamine receptors are classified into different subtypes, including D1, D2, D3, D4, and D5 receptors. DRDs antagonists can selectively target specific subtypes of dopamine receptors or have a broader spectrum of activity across multiple subtypes.
Overall, DRDs antagonists are a class of drugs that act by blocking dopamine receptors and are used in the management of various psychiatric disorders.
Drug Target R&D Trends for Thiothixene
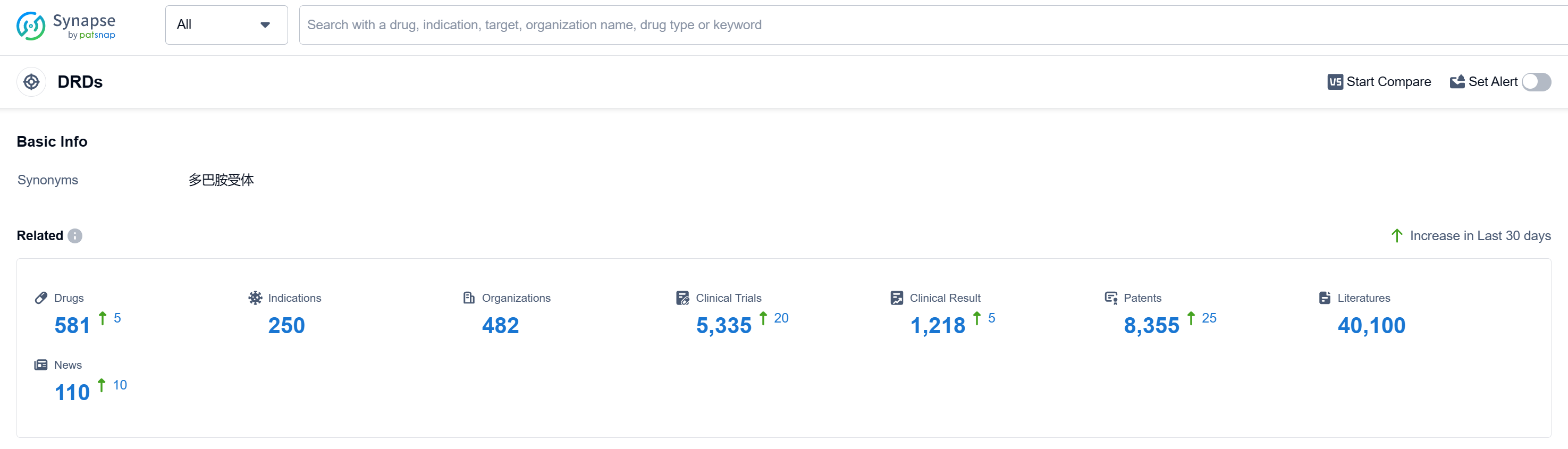
According to Patsnap Synapse, as of 12 Sep 2023, there are a total of 581 DRDs drugs worldwide, from 482 organizations, covering 250 indications, and conducting 5335 clinical trials.
Based on the analysis of the provided data, the current competitive landscape of target DRDs is characterized by the active involvement of various pharmaceutical companies, with Johnson & Johnson, Novartis AG, and Mitsubishi Chemical Group Corp. leading in terms of growth and R&D progress. The highest stage of development is the "Approved" phase, indicating successful regulatory approval of drugs. The most common indications for approved drugs include Schizophrenia, Parkinson Disease, Bipolar Disorder, and Nausea. Small molecule drugs dominate the drug types progressing rapidly under the target DRDs. China, the United States, and Japan are the countries/locations developing fastest, with China showing significant progress. Overall, the target DRDs present a competitive landscape with diverse companies, indications, drug types, and global development.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Thiothixene is a small molecule drug developed by Pfizer Inc. It targets DRDs and is primarily used in the therapeutic area of Other Diseases. With its first approval dating back to 1967 in the United States, Thiothixene has a long-standing history in the pharmaceutical industry. However, without further information, it is challenging to provide a more detailed analysis of its specific indications and clinical applications.