Deep Scientific Insights on Vosoritide's R&D Progress, Mechanism of Action
Vosoritide's R&D Progress
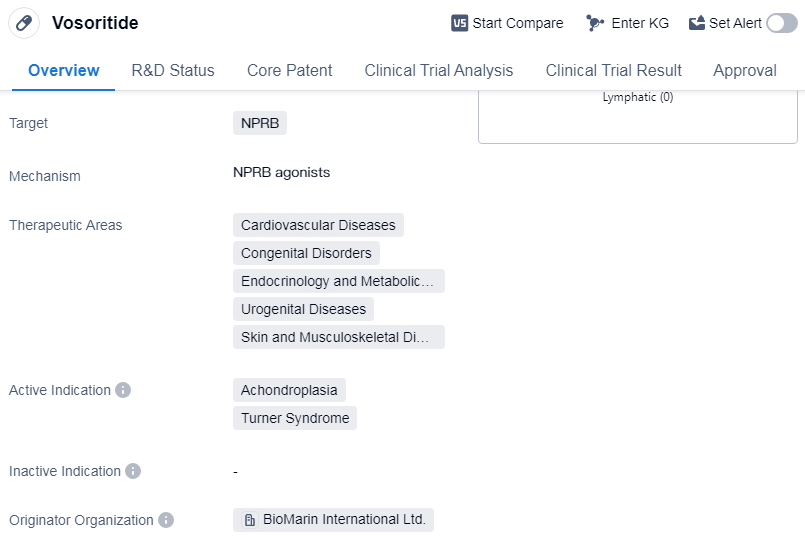
Vosoritide is a recombinant polypeptide drug that targets NPRB. It falls under various therapeutic areas, including cardiovascular diseases, congenital disorders, endocrinology and metabolic disease, urogenital diseases, and skin and musculoskeletal diseases. The drug is primarily indicated for the treatment of achondroplasia and Turner syndrome.
BioMarin International Ltd. is the originator organization of Vosoritide. As of the highest phase, the drug has been approved globally. The first approval of Vosoritide was granted in August 2021, and it was approved in multiple countries, including the European Union, Iceland, Liechtenstein, and Norway.
In terms of regulation, Vosoritide has undergone priority review and accelerated approval processes. It has also been designated as an orphan drug. These regulatory designations aim to expedite the development and approval of drugs that address rare diseases or conditions.
Achondroplasia is a genetic disorder that affects bone growth, resulting in dwarfism. Turner syndrome, on the other hand, is a chromosomal condition that affects females and leads to short stature and other health issues. Vosoritide's approval for these indications suggests its potential in addressing the underlying causes and symptoms of these conditions.
Being a recombinant polypeptide, Vosoritide is a biologic drug that is produced through genetic engineering techniques. This type of drug is known for its complex manufacturing process and high specificity in targeting specific receptors or molecules in the body.
The approval of Vosoritide in multiple countries and its designation as an orphan drug highlight the unmet medical need and potential therapeutic value it offers for patients with achondroplasia and Turner syndrome. The drug's mechanism of action, targeting NPRB, indicates its potential in modulating the signaling pathways involved in bone growth and development.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Vosoritide: NPRB agonists
NPRB agonists are a type of drug that activate the NPRB receptor. The NPRB receptor, also known as the natriuretic peptide receptor B, is a protein found on the surface of certain cells in the body. When activated by NPRB agonists, this receptor initiates a signaling cascade that leads to various physiological effects.
From a biomedical perspective, NPRB agonists are of interest because they can modulate the activity of the natriuretic peptide system, which plays a crucial role in regulating blood pressure, fluid balance, and cardiovascular function. The natriuretic peptide system consists of a family of hormones called natriuretic peptides, which are released by the heart and other organs in response to changes in blood volume and pressure.
By activating the NPRB receptor, NPRB agonists mimic the effects of natriuretic peptides, leading to vasodilation (widening of blood vessels), diuresis (increased urine production), and natriuresis (increased excretion of sodium in urine). These effects help to reduce blood pressure and promote fluid balance in the body.
NPRB agonists have potential therapeutic applications in the treatment of cardiovascular diseases such as hypertension (high blood pressure) and heart failure. By targeting the NPRB receptor, these agonists can enhance the activity of the natriuretic peptide system and improve cardiovascular function.
It's important to note that the specific mechanism of action and clinical applications of NPRB agonists may vary depending on the specific compound being used. Further research and clinical trials are needed to fully understand the potential benefits and limitations of NPRB agonists in biomedicine.
Drug Target R&D Trends for Vosoritide
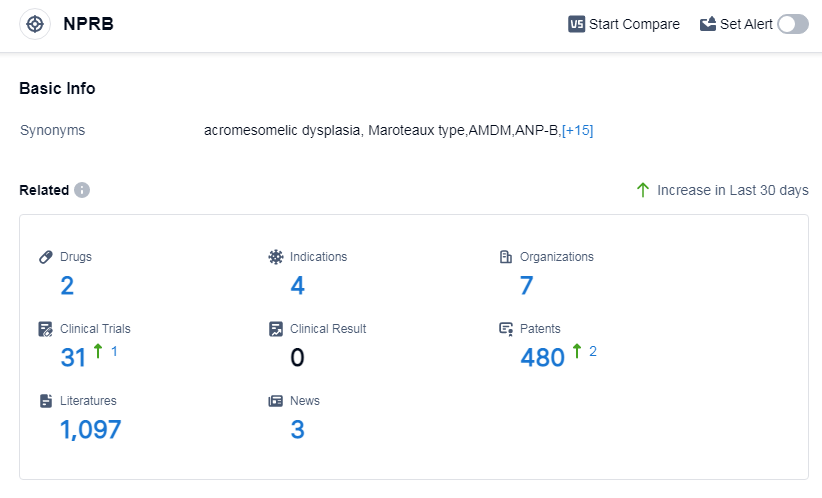
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 2 NPRB drugs worldwide, from 7 organizations, covering 4 indications, and conducting 31 clinical trials.
Based on the analysis of the provided data, the current competitive landscape for the target NPRB is characterized by the active involvement of companies such as BioMarin Pharmaceutical, Inc., BioMarin Pharmaceutical Japan K.K., Children's National Research Institute, Mayo Clinic, and Capricor Therapeutics, Inc. These companies are driving R&D progress in various phases, indicating a strong focus on the target NPRB. Drugs have been approved for indications such as achondroplasia, and ongoing development is observed for indications like Turner Syndrome. The presence of biosimilars and the development of different drug types, including recombinant polypeptide and synthetic peptide, suggest intense competition and innovation in the pharmaceutical industry. The countries/locations with the highest phase under the target NPRB include the European Union, United States, Liechtenstein, Iceland, Japan, Norway, Australia, Brazil, Germany, Spain, United Kingdom, Turkey, France, Israel, and Russia. This global effort signifies the widespread interest and investment in the target NPRB. However, the lack of information about China's progress suggests a relatively slower development in this country. Overall, the target NPRB shows promising growth potential and a competitive landscape in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Vosoritide's approval and therapeutic indications make it a significant advancement in the field of biomedicine, particularly in addressing rare genetic disorders affecting bone growth and development. Its regulatory designations and originator organization further emphasize the importance and potential impact of this drug in the pharmaceutical industry.