Desonide: Detailed Review of its Transformative R&D Success, Mechanism of Action
Desonide's R&D Progress
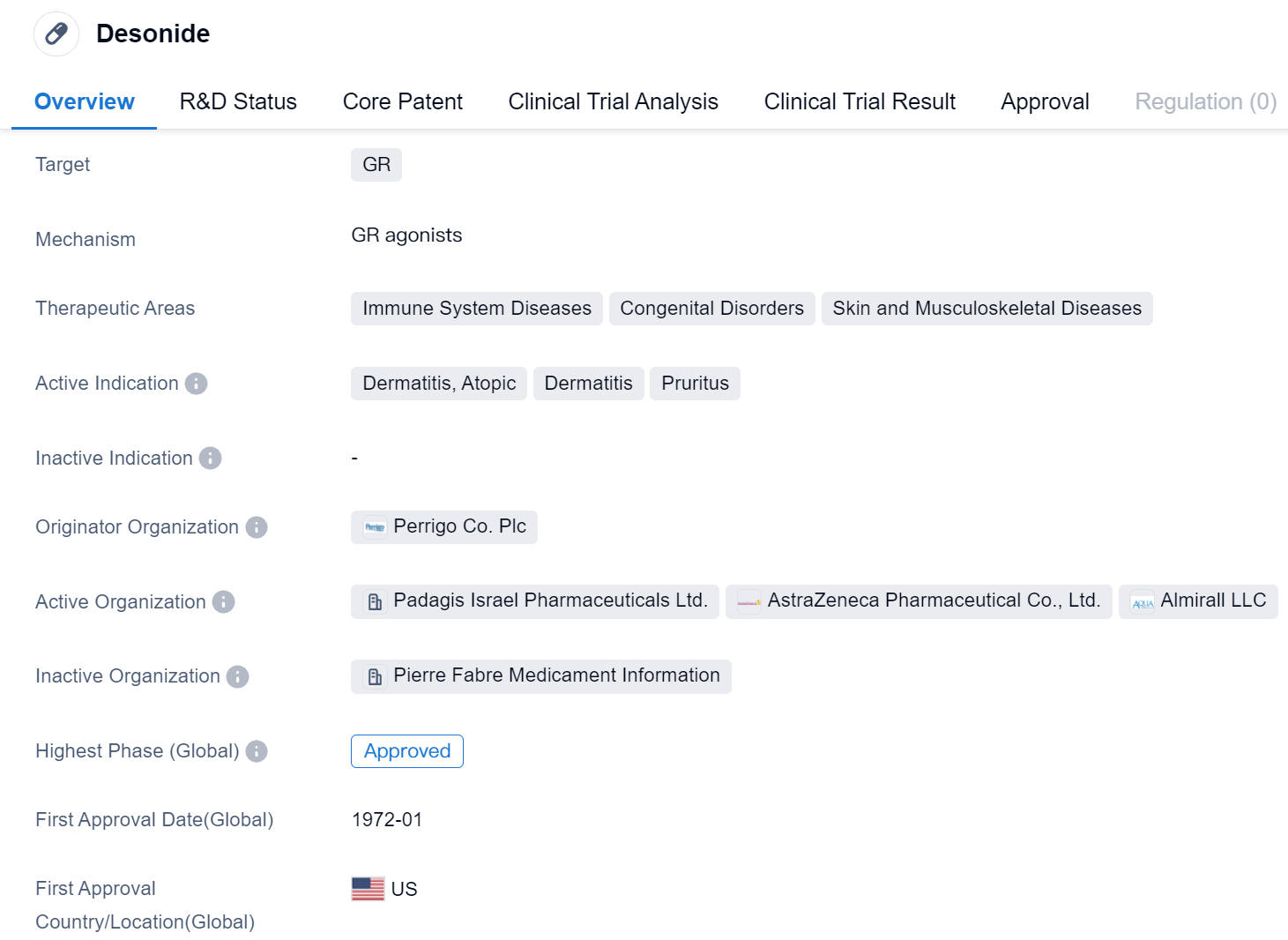
Desonide is a small molecule drug that targets the glucocorticoid receptor (GR). It is primarily used in the treatment of immune system diseases, congenital disorders, and skin and musculoskeletal diseases. The active indications for Desonide include dermatitis, atopic dermatitis, and pruritus.
Desonide was first approved in the United States in January 1972, making it a well-established drug in the pharmaceutical market. It is important to note that Desonide has received approval in global market, indicating its widespread use and recognition.
The originator organization of Desonide is Perrigo Co. Plc, a pharmaceutical company known for its expertise in developing and manufacturing a wide range of healthcare products. As the originator, Perrigo Co. Plc holds the intellectual property rights and is responsible for the initial development and commercialization of Desonide.
Desonide's therapeutic areas highlight its versatility in treating various medical conditions. Immune system diseases, such as autoimmune disorders, can benefit from Desonide's ability to modulate the immune response. Congenital disorders, which are present from birth, may also be managed with Desonide. Additionally, Desonide is effective in treating skin and musculoskeletal diseases, including dermatitis and pruritus.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Desonide: GR agonists
GR agonists refer to agonists of the glucocorticoid receptor (GR). The glucocorticoid receptor is a protein found in cells that binds to glucocorticoid hormones, such as cortisol, and plays a crucial role in regulating various physiological processes, including metabolism, inflammation, and immune response. GR agonists are compounds or drugs that activate the glucocorticoid receptor, mimicking the effects of endogenous glucocorticoids.
From a biomedical perspective, GR agonists are commonly used in the treatment of inflammatory and autoimmune diseases, such as asthma, rheumatoid arthritis, and inflammatory bowel disease. By activating the glucocorticoid receptor, these agonists can suppress inflammation and modulate the immune response, leading to reduced symptoms and improved clinical outcomes. However, long-term use of GR agonists can have side effects, including adrenal suppression, osteoporosis, and metabolic disturbances, which need to be carefully monitored.
Drug Target R&D Trends for Desonide
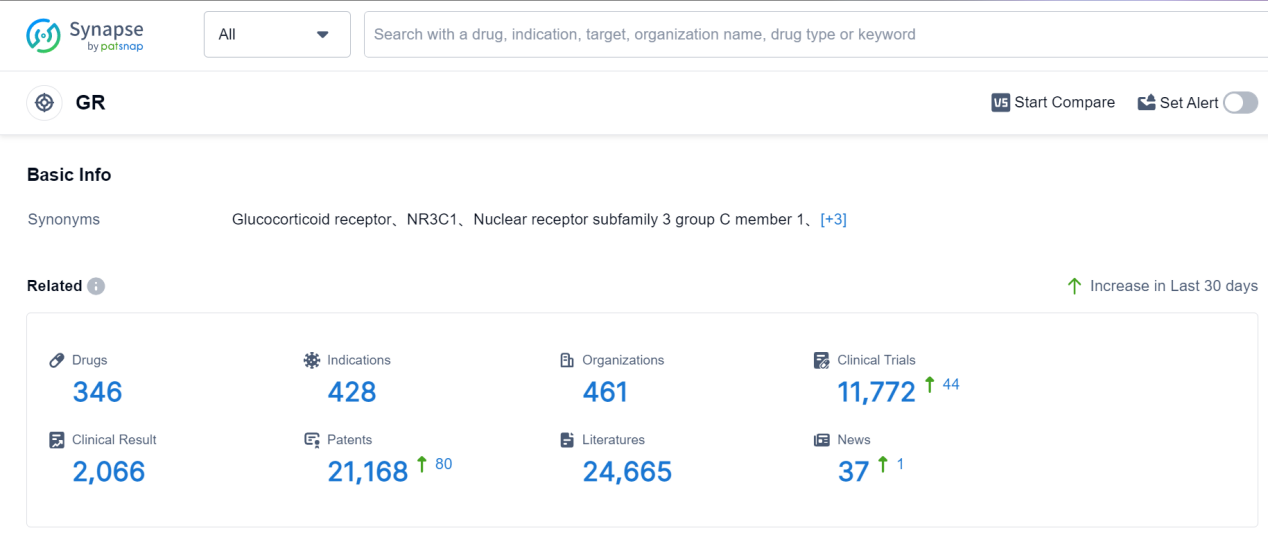
GR, or glucocorticoid receptor, plays a crucial role in the human body. It is a protein found in cells that binds to glucocorticoid hormones, such as cortisol. Once activated, GR regulates various physiological processes, including metabolism, immune response, and stress management. GR acts as a transcription factor, influencing gene expression and controlling the production of proteins involved in these processes. It helps maintain homeostasis by modulating inflammation, glucose metabolism, and the body's response to stress. Dysregulation of GR function has been associated with various diseases, highlighting its importance in maintaining overall health and well-being.
According to Patsnap Synapse, as of 7 Sep 2023, there are a total of 346 GR drugs worldwide, from 461 organizations, covering 428 indications, and conducting 11772 clinical trials.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Desonide is a small molecule drug that targets the glucocorticoid receptor. It has been approved for use in the treatment of immune system diseases, congenital disorders, and skin and musculoskeletal diseases. With its active indications being dermatitis, atopic dermatitis, and pruritus, Desonide has gained recognition and approval in both the global markets. Perrigo Co. Plc is the originator organization responsible for the development and commercialization of Desonide. Its long history of approval since 1972 demonstrates its established position in the pharmaceutical industry.