Domvanalimab and Zimberelimab Improve Survival in ARC-10 Lung Cancer Trial
Arcus Biosciences, Inc. (NYSE:RCUS), a clinical-stage biopharmaceutical firm dedicated to the development of unique molecules and combination therapies for cancer patients, has shared findings from Part 1 of the ARC-10 trial. This randomized, open-label, three-arm study assesses the efficacy of domvanalimab, an Fc-silent anti-TIGIT monoclonal antibody, in combination with zimberelimab, an anti-PD-1 monoclonal antibody (referred to as DZ), compared to zimberelimab alone (Z) or chemotherapy. The trial targeted patients with newly diagnosed locally advanced or metastatic squamous or non-squamous non-small cell lung cancer (NSCLC) exhibiting a PD-L1 tumor proportion score (TPS) of 50% or higher, and no tumor genomic alterations or driver mutations that would qualify for approved targeted therapies. This research was conducted in collaboration with Gilead Sciences. The results will be showcased in a late-breaking poster session on November 8 at the Society for Immunotherapy of Cancer (SITC) 2024 Annual Meeting, presented by Dr. Melissa L. Johnson, Director of the Lung Cancer Research Program at the Sarah Cannon Research Institute and an investigator of the ARC-10 study.
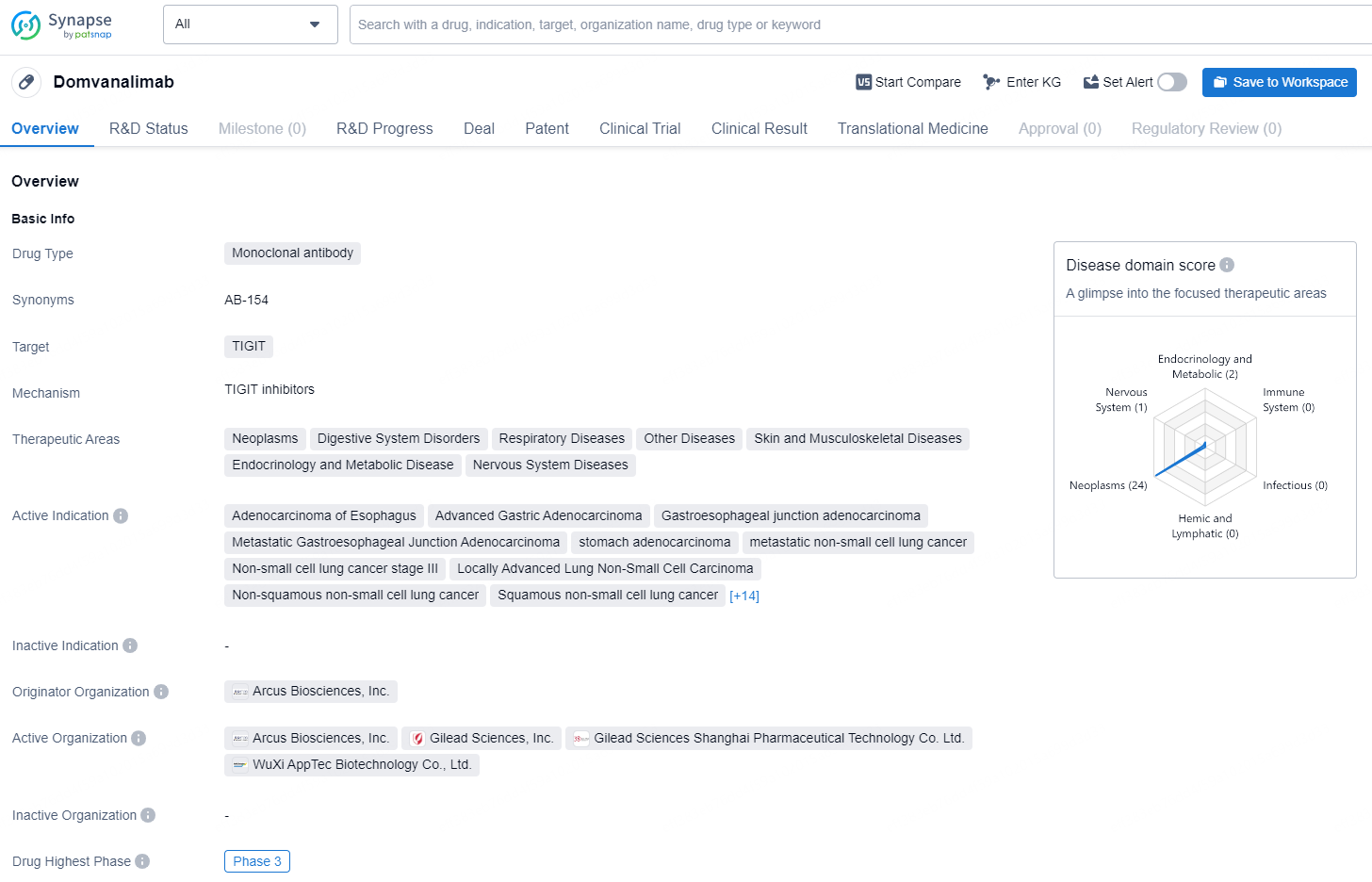
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“Combining domvanalimab with zimberelimab showed a significant enhancement in overall survival rates when compared to zimberelimab alone, resulting in a 36% decrease in mortality risk, with median overall survival expected to surpass two years,” stated Melissa L. Johnson, M.D. “These findings further support the notion that simultaneous inhibition of the TIGIT and PD-1 pathways may provide a superior therapeutic advantage compared to targeting the PD-1 pathway in isolation.”
“These are the initial findings indicating an increase in overall survival for domvanalimab in combination with zimberelimab,” commented Dimitry Nuyten, M.D., Ph.D., chief medical officer of Arcus. “These results contribute to the expanding evidence that domvanalimab, an Fc-silent anti-TIGIT monoclonal antibody, may exhibit distinct efficacy, safety, and tolerability characteristics in comparison to data from studies involving Fc-enabled anti-TIGIT antibodies.”
As of the data cutoff (DCO, May 17, 2024), a total of 98 patients were randomized, with 95 receiving treatment in the first part of the study. The median follow-up duration was 24.5 months; at the time of the DCO, 22 patients continued to receive treatment (DZ, n=11; Z, n=10; chemo, n=1). Baseline demographic characteristics of patients were largely balanced across the treatment groups, although there was a slight imbalance with a higher number of adenocarcinoma cases, ECOG status 0, and brain metastasis in the chemotherapy group. A summary of the efficacy findings follows.
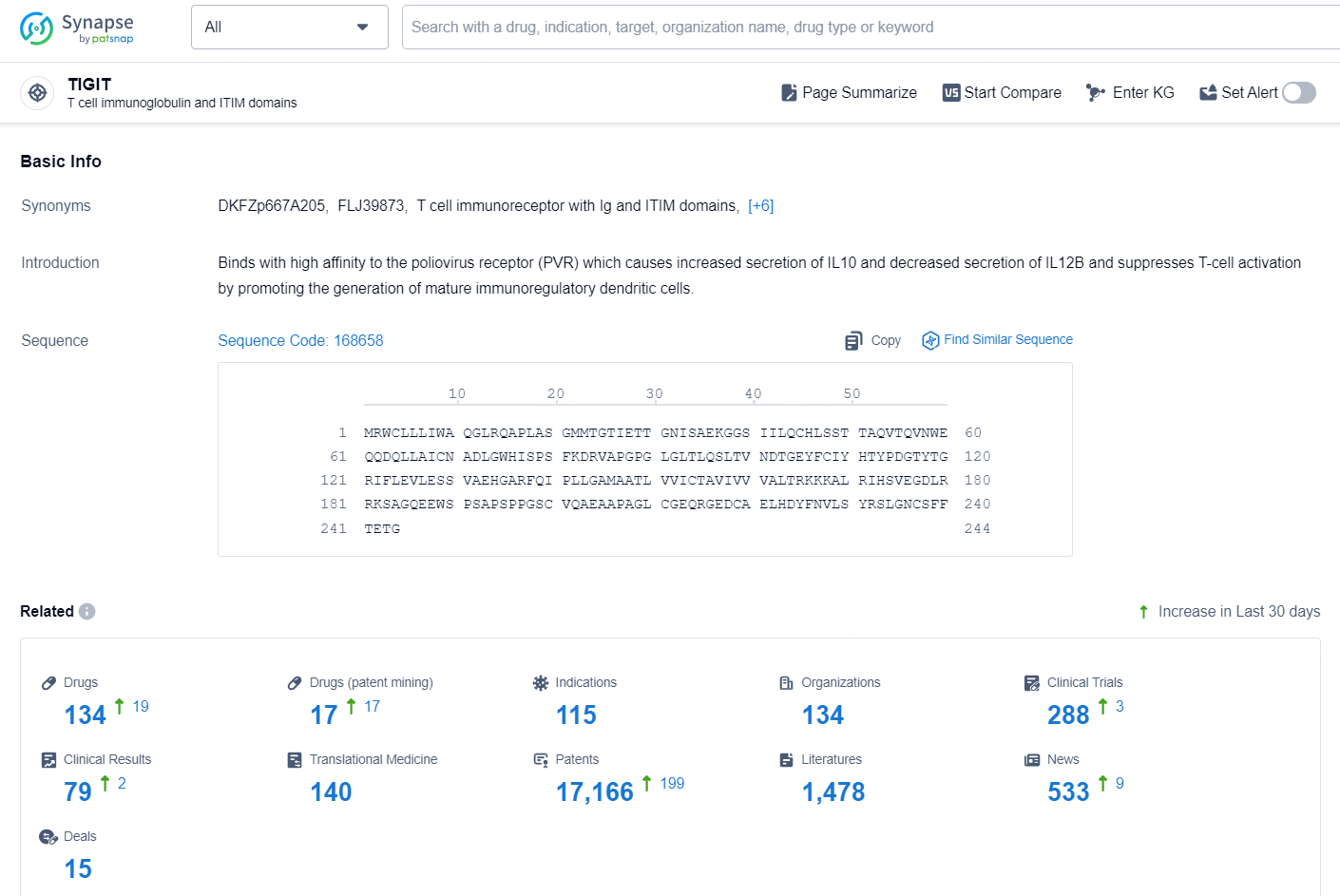
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of November 7, 2024, there are 134 investigational drugs for the TIGIT target, including 115 indications, 134 R&D institutions involved, with related clinical trials reaching 288, and as many as 17166 patents.
Domvanalimab is a monoclonal antibody drug that targets TIGIT, a protein typically found on the surface of immune cells. This drug is being developed for various therapeutic areas, including neoplasms, digestive system disorders, respiratory diseases, skin and musculoskeletal diseases, endocrinology and metabolic disease, and nervous system diseases.