OSE Immunotherapeutics Announces Significant Phase 2 Results for Lusvertikimab in Ulcerative Colitis Treatment
OSE Immunotherapeutics SA has announced encouraging findings from the induction phase of the CoTikiS randomized, double-blind, placebo-controlled Phase 2 trial involving Lusvertikimab (OSE-127). The results indicate significant effectiveness and a positive safety profile in patients suffering from moderate to severe active ulcerative colitis (UC).
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Summary of Findings from the CoTikiS Phase 2 Trial
The CoTikiS Phase 2 randomized, double-blind clinical trial investigated the effectiveness and safety of Lusvertikimab in comparison to placebo in 136 individuals with moderate to severe active ulcerative colitis (UC) who either did not respond to or lost their response to earlier therapies*. This study spans 50 weeks and includes a 10-week induction phase where two dosages of Lusvertikimab (450 mg and 850 mg) are evaluated against placebo, followed by a 24-week open-label extension period (OLE) where all participants receive 850 mg of Lusvertikimab every four weeks, and concludes with a 16-week follow-up phase without treatment.
Lusvertikimab (Lusv) successfully achieved the main efficacy criterion, which was assessed through the Modified Mayo Score (MMS) at week 10 (W10), yielding statistically significant and clinically relevant outcomes on key secondary measures at both tested doses. Throughout the induction phase as well as the subsequent six-month open-label extension, a positive safety profile was noted. In the W0-W10 phase, data from 134 patients were included in the analysis [group receiving 850 mg (50 patients); group receiving 450 mg (35 patients); combined drug group (85 patients); placebo group (49 patients)]. Additionally, 120 patients who received Lusvertikimab engaged in the 24-week OLE treatment phase.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
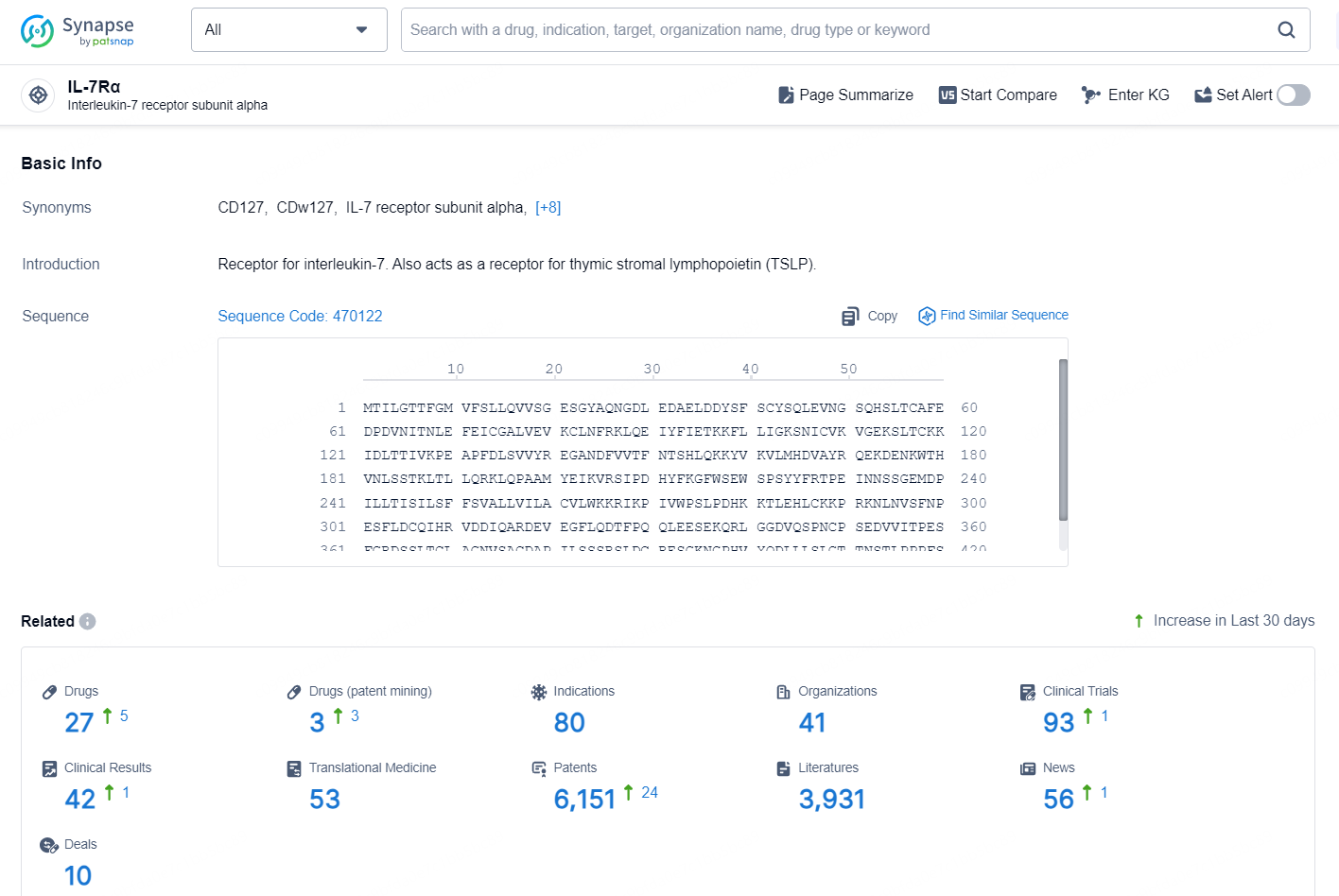
According to the data provided by the Synapse Database, As of November 7, 2024, there are 27 investigational drug for the IL-7Rα target, including 80 indications, 41 R&D institutions involved, with related clinical trials reaching 93, and as many as 6151 patents.
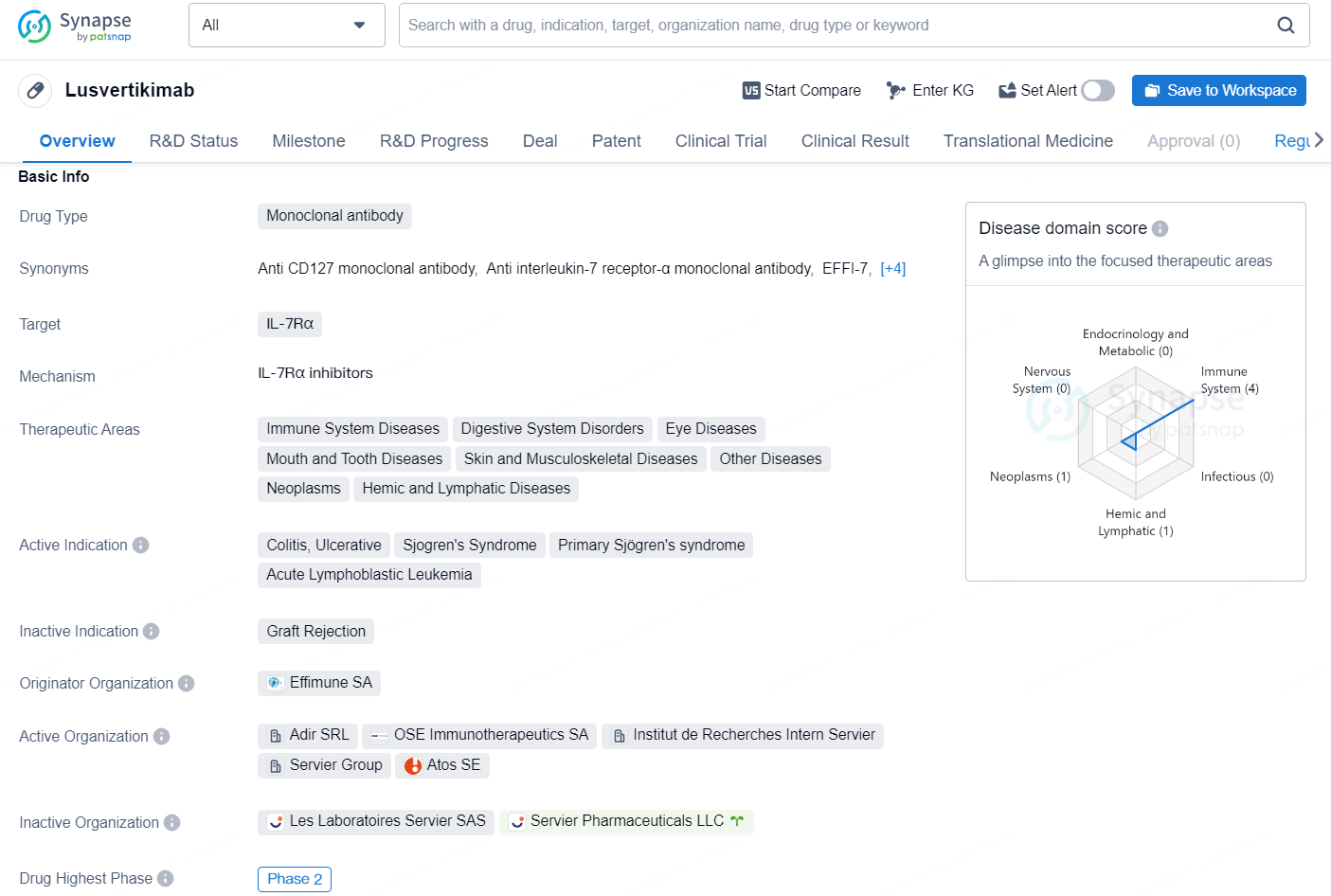
Lusvertikimab is a monoclonal antibody drug that targets IL-7Rα and is currently in the highest global phase of Phase 2. This drug is being developed by Effimune SA, and it is intended to be used in the treatment of a wide range of therapeutic areas, including immune system diseases, digestive system disorders, eye diseases, mouth and tooth diseases, skin and musculoskeletal diseases, neoplasms, and hemic and lymphatic diseases. The active indications for Lusvertikimab include colitis, ulcerative Sjogren's syndrome, primary Sjögren's syndrome, and acute lymphoblastic leukemia.