European Medicines Agency Approves Marketing Authorization Application for AVT05
Alvotech (NASDAQ: ALVO), a worldwide biotechnology firm focused on the creation and production of biosimilar pharmaceuticals for patients globally, in partnership with Advanz Pharma, a UK-based global pharmaceutical entity with a dedicated approach to specialty, hospital, and medicines for rare diseases in Europe, has revealed that the European Medicines Agency (EMA) has accepted a Marketing Authorization Application for AVT05. This product is Alvotech's proposed biosimilar to Simponi® (golimumab), a biologic that treats various chronic inflammatory conditions. This submission is thought to be the first announcement on a global scale for a biosimilar candidate targeting Simponi. The review process for approval is expected to be concluded by the fourth quarter of 2025.
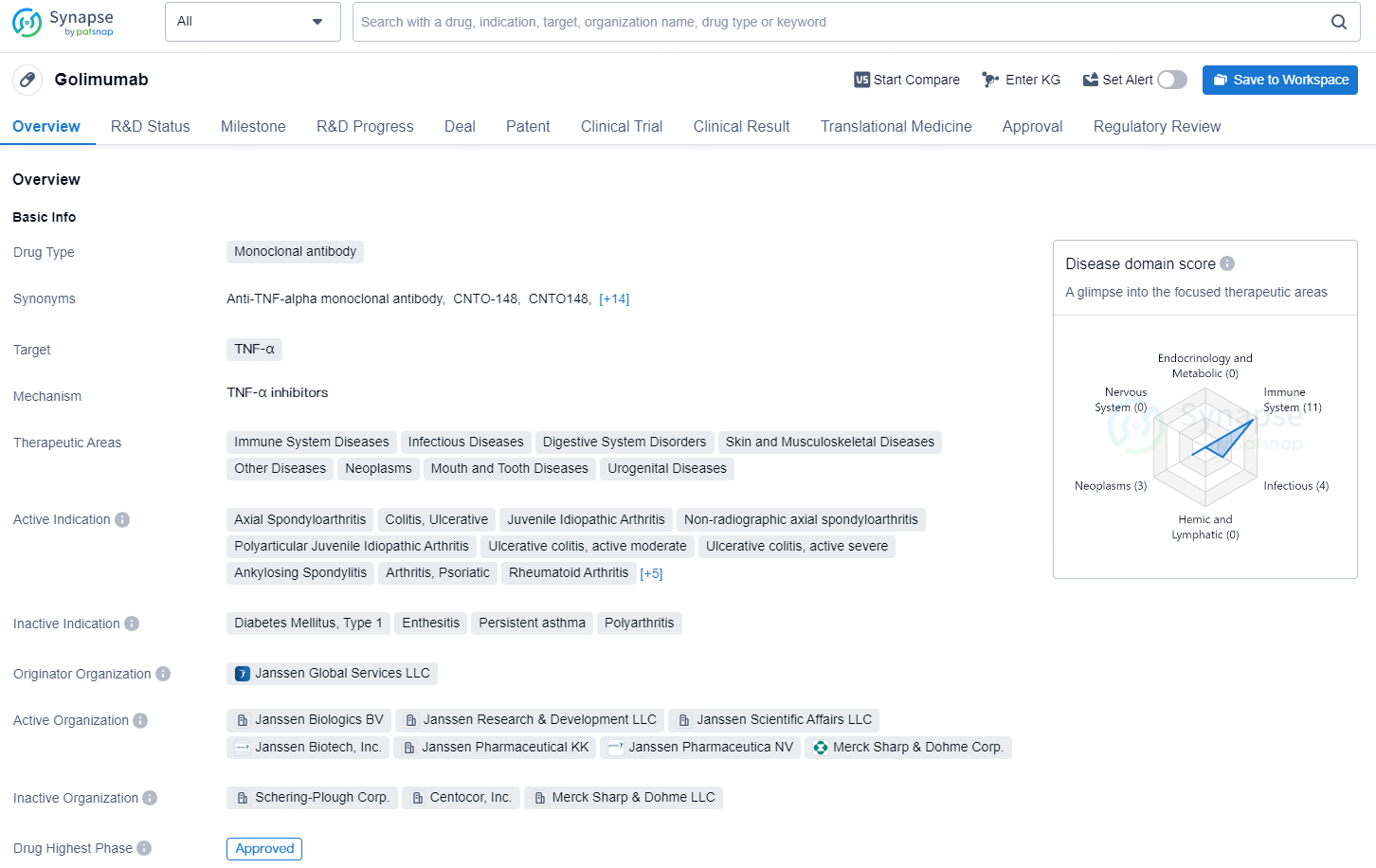
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"This achievement marks a significant milestone for us, alongside our partners, patients, and caregivers, as we move closer to providing access to the biosimilar Simponi®,” stated Joseph McClellan, Chief Scientific Officer at Alvotech. “We are confident that our internal capabilities and expertise in utilizing a host cell line and the same manufacturing process as the reference biologic have given us a crucial advantage in developing a biosimilar candidate for Simponi®."
“The European Medicines Agency's acceptance of the AVT05 application is a considerable advancement in broadening treatment alternatives for individuals with chronic inflammatory disorders throughout Europe,” remarked Nick Warwick, Chief Medical Officer at Advanz Pharma. “We are dedicated to enhancing patient access to high-quality biologic therapies.”
In February 2023, Alvotech and Advanz Pharma announced their collaboration on a commercialization agreement for AVT23, a proposed biosimilar to Xolair® (omalizumab). In May of the same year, the partners revealed an extension of their strategic alliance to also include five additional biosimilar candidates being developed by Alvotech, including AVT05, AVT16—proposed as a biosimilar to Entyvio® (vedolizumab)—and three other early-stage biosimilar candidates that have yet to be disclosed.
In April 2024, Alvotech reported positive top-line outcomes from a confirmatory clinical trial that compared the efficacy, safety, and immunogenicity of AVT05 with Simponi® in patients suffering from moderate to severe rheumatoid arthritis. Additionally, in November 2023, Alvotech announced favorable top-line data from a pharmacokinetic study investigating the pharmacokinetics, safety, and tolerability of AVT05 in healthy adult participants compared to Simponi®.
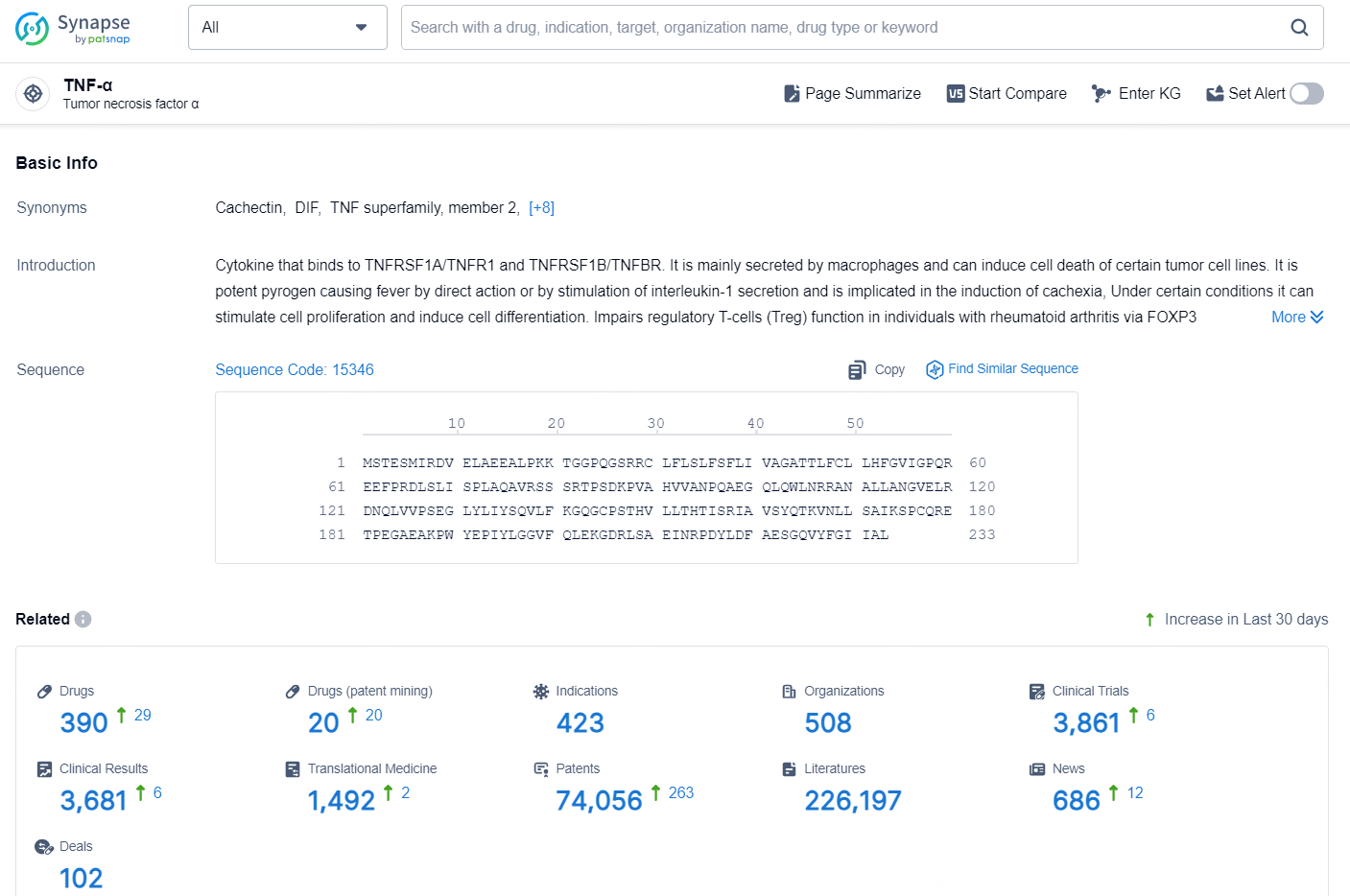
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of November 7, 2024, there are 390 investigational drugs for the TNF-α target, including 423 indication, 508 R&D institutions involved, with related clinical trial reaching 3861, and as many as 74056 patents.
Golimumab is a monoclonal antibody drug that targets TNF-α and is used in the treatment of various therapeutic areas including immune system diseases, infectious diseases, digestive system disorders, skin and musculoskeletal diseases, neoplasms, mouth and tooth diseases, urogenital diseases, and other diseases.