Advancements in Anemia Treatment for Chronic Kidney Disease: The Role of Daprodustat
Daprodustat is a small molecule drug developed by GSK Plc which primarily targets Hypoxia-Inducible Factor-Prolyl Hydroxylase (HIF-PH) enzymes. This drug falls under the therapeutic areas of Hemic and Lymphatic Diseases, as well as Urogenital Diseases. Its active indication is for the treatment of anemia in chronic kidney disease and chronic kidney diseases. The global highest phase of development for Daprodustat is 'Approved', with the first approval occurring in June 2020. The initial approval took place in Japan, indicating that the drug has successfully completed the regulatory approval process in that country.
The approval of Daprodustat represents a significant milestone in the treatment of anemia in chronic kidney disease. As a small molecule drug, it is designed to mimic the body's natural response to low oxygen levels, thereby stimulating the production of red blood cells and increasing hemoglobin levels. This mechanism of action makes Daprodustat a potentially valuable therapeutic option for patients with anemia associated with chronic kidney disease, providing an alternative to traditional erythropoiesis-stimulating agents.
Additionally, the approval of Daprodustat underscores GSK Plc's continued commitment to addressing unmet medical needs in the field of biomedicine. The drug's approval in Japan reflects the company's successful navigation of the regulatory process in a key market, paving the way for potential approvals in other regions.
Below, we will use the drug Daprodustat as an example to demonstrate how to quickly obtain information about its chemical structure and patent situation using the Patsnap Chemical.
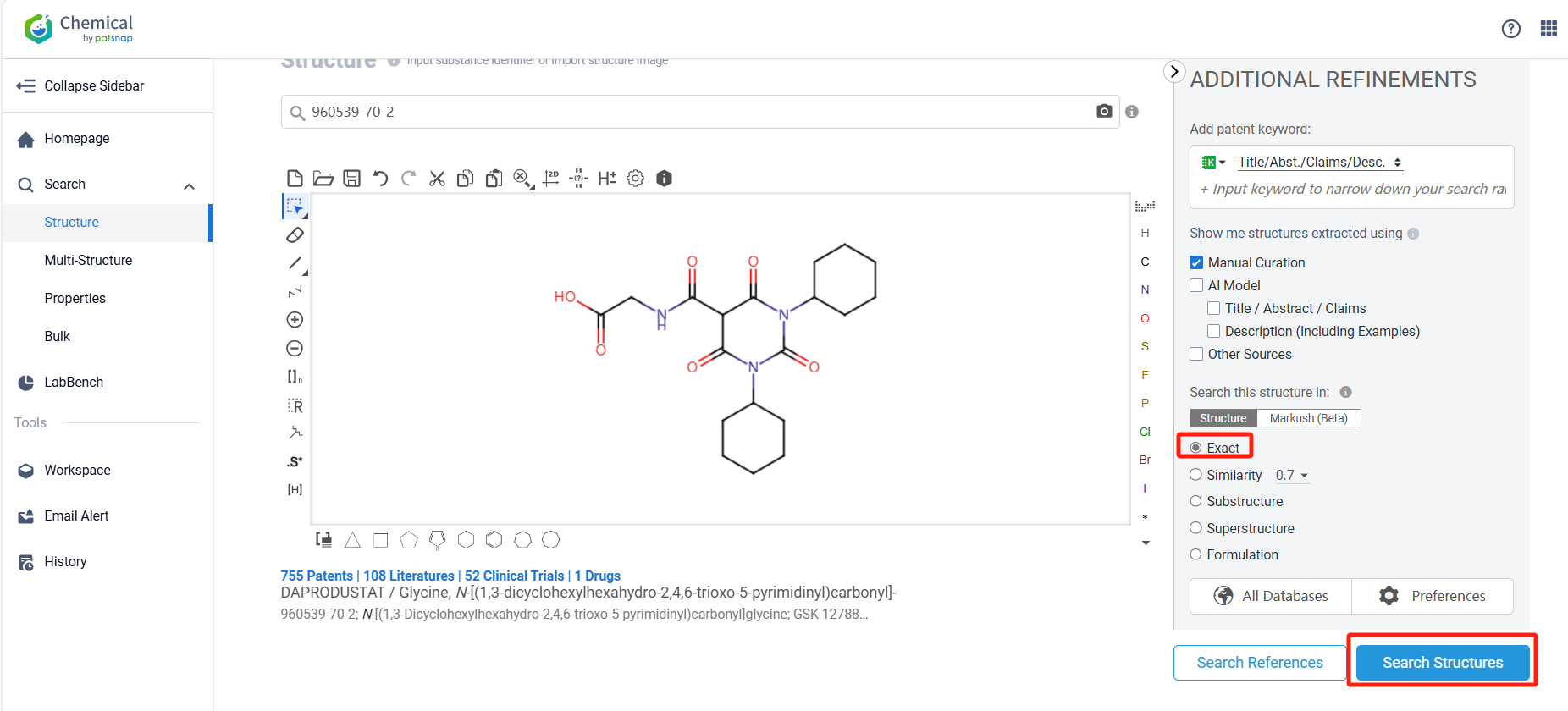
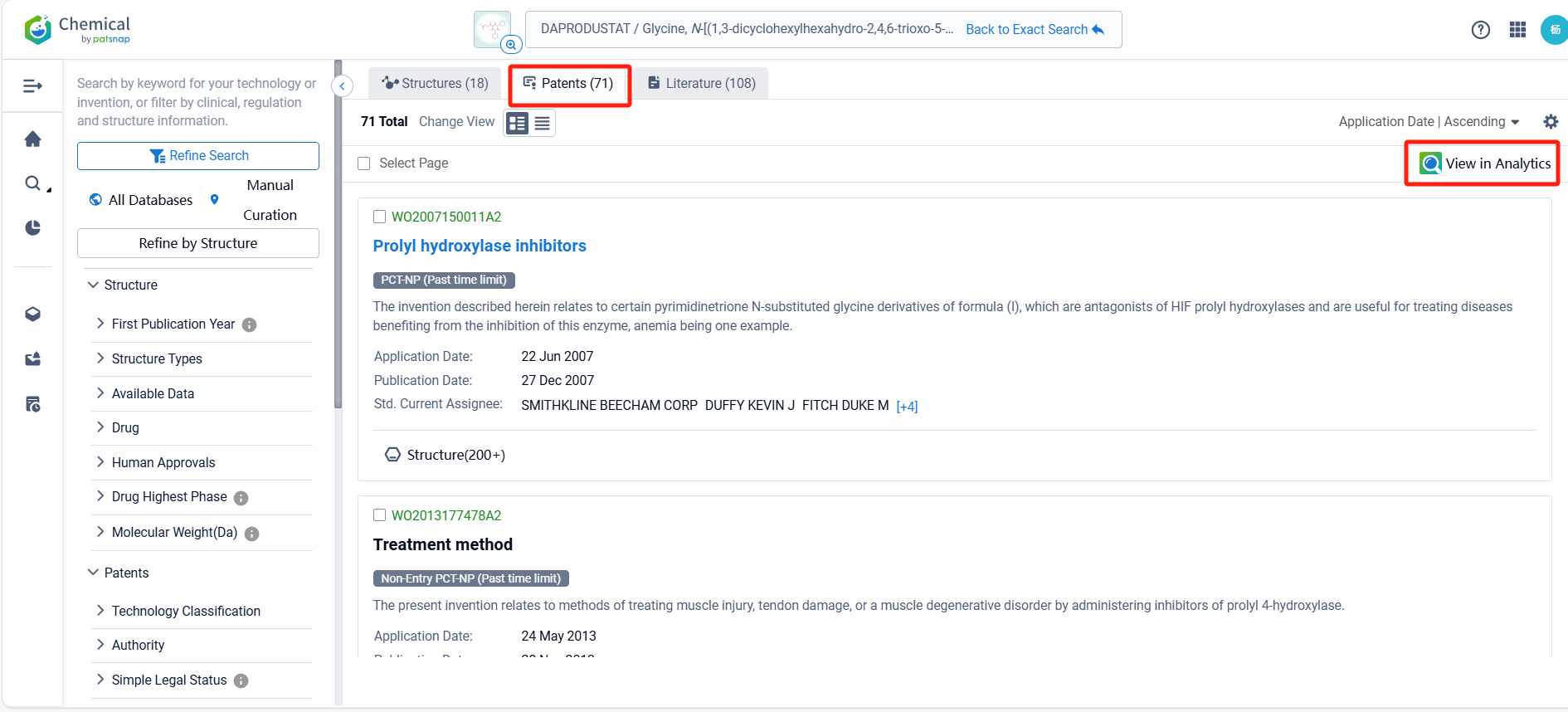
Log in to the Patsnap Chemical. Select the structural search and enter the common identity information of Daprodustat (such as CAS number, generic substance name, molecular formula, SMILES file, etc.). Here, we select "Exact Search", click on search structures, and you can find 71 patents. Clicking the "view in Analytics" will direct you to the Patsnap Patent.
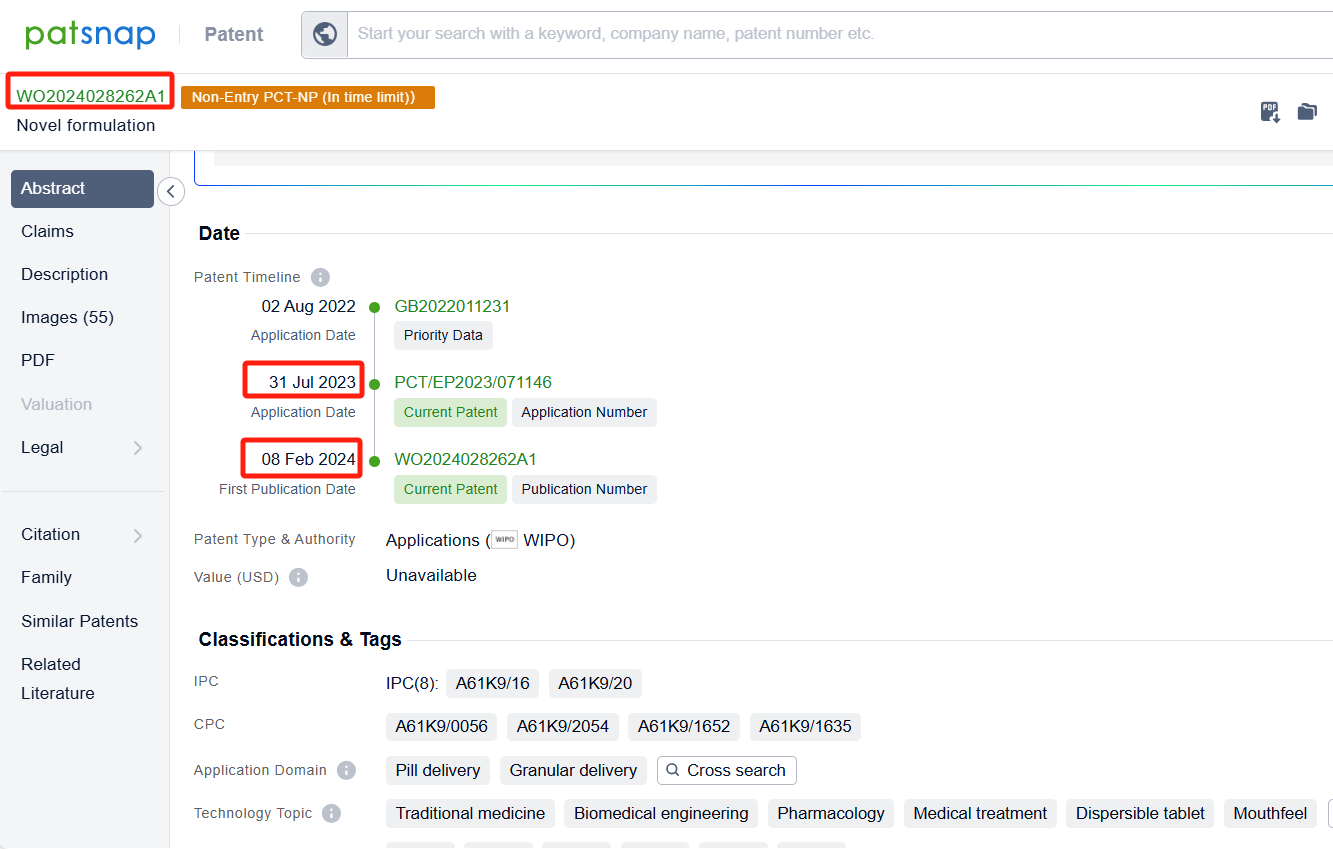
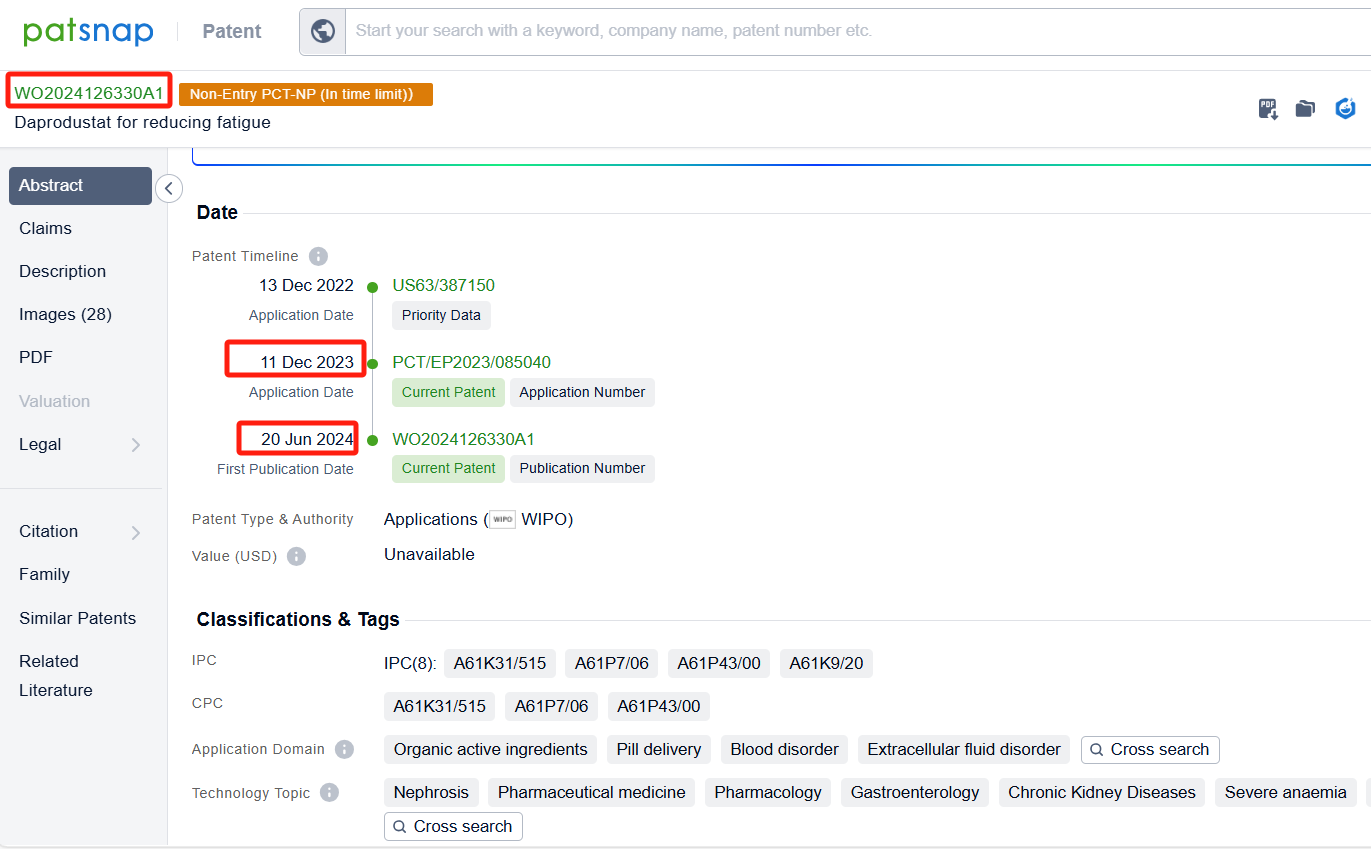
In the Patsnap patent database, we can sort patents by their publication dates to identify the latest patents on Daprodustat. By reviewing the aforementioned patents, we can observe that GlaxoSmithKline Intellectual Property (No.2) Ltd.'s international patent WO2024028262A1 (application date 20230731, publication date 20240208) is to provide a dispersible tablet of Daprodustat, The present disclosure relates to a dispersible tablet capable of disintegrating in very small volumes of water rapidly to form a suspension that is free flowing and has an acceptable mouthfeel. Additionally, GlaxoSmithKline Intellectual Property (No.2) Ltd.'s patent WO2024126330A1 (application date 20231211, publication date 20240620) describes the use of daprodustat or a pharmaceutically acceptable salt thereof to reduce fatigue in subjects with anemia associated with chronic kidney disease who are not on dialysis and have hepcidin levels above a certain threshold.
 In summary, Daprodustat is a small molecule drug developed by GSK Plc, targeting HIF-PHs for the treatment of anemia in chronic kidney disease. With its approval in Japan in June 2020, this drug represents a significant advancement in addressing unmet medical needs in the field of biomedicine and offering new therapeutic options for patients with chronic kidney disease.
In summary, Daprodustat is a small molecule drug developed by GSK Plc, targeting HIF-PHs for the treatment of anemia in chronic kidney disease. With its approval in Japan in June 2020, this drug represents a significant advancement in addressing unmet medical needs in the field of biomedicine and offering new therapeutic options for patients with chronic kidney disease.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.