Alnylam Submits Vutrisiran Application to EMA for ATTR Amyloidosis with Cardiomyopathy
Alnylam Pharmaceuticals, Inc. (Nasdaq: ALNY), a leading company in RNA interference (RNAi) therapeutics, has announced the filing of a Type II Variation with the European Medicines Agency (EMA) for vutrisiran, an investigational RNAi therapy aimed at treating ATTR amyloidosis with cardiomyopathy (ATTR-CM). vutrisiran, the generic designation for AMVUTTRA®, is presently authorized in the European Union (EU) for managing hereditary transthyretin-mediated (hATTR) amyloidosis in adult patients experiencing stage 1 or stage 2 polyneuropathy.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"Today signifies yet another significant step in our mission to deliver RNAi therapeutics to patients across the globe with substantial unmet medical needs," stated Pushkal Garg, M.D., the Chief Medical Officer of Alnylam. "ATTR-CM is a rapidly advancing, disabling, and potentially fatal condition that is being increasingly recognized as a contributing factor to heart failure. Vutrisiran effectively reduces TTR levels, and in the HELIOS-B study, treatment with vutrisiran led to a notable decrease in overall mortality and cardiovascular events, highlighting the promise of this therapy for those affected by the disease. We are eager to collaborate closely with the EMA to expedite the availability of this new treatment option for patients."
The regulatory submission is grounded in the favorable outcomes from the pivotal HELIOS-B Phase 3 study, which was randomized, double-blind, placebo-controlled, and conducted across multiple centers globally. It successfully achieved all 10 primary and secondary endpoints with statistical significance in both the overall population and monotherapy group.
The results illustrated the impact of vutrisiran on mortality rates, cardiovascular events, as well as functional ability (measured by the 6-minute walk test), quality of life (assessed through the Kansas City Cardiomyopathy Questionnaire), and symptoms and severity of heart failure (evaluated using the NYHA classification) in patients diagnosed with ATTR-CM. The safety profile of vutrisiran observed in HELIOS-B was in line with its known safety record for hATTR amyloidosis treatment in adult patients experiencing polyneuropathy. In this study, the incidence of adverse events (AEs), serious adverse events, severe AEs, and AEs resulting in discontinuation of the study drug were comparable between the vutrisiran and placebo groups.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
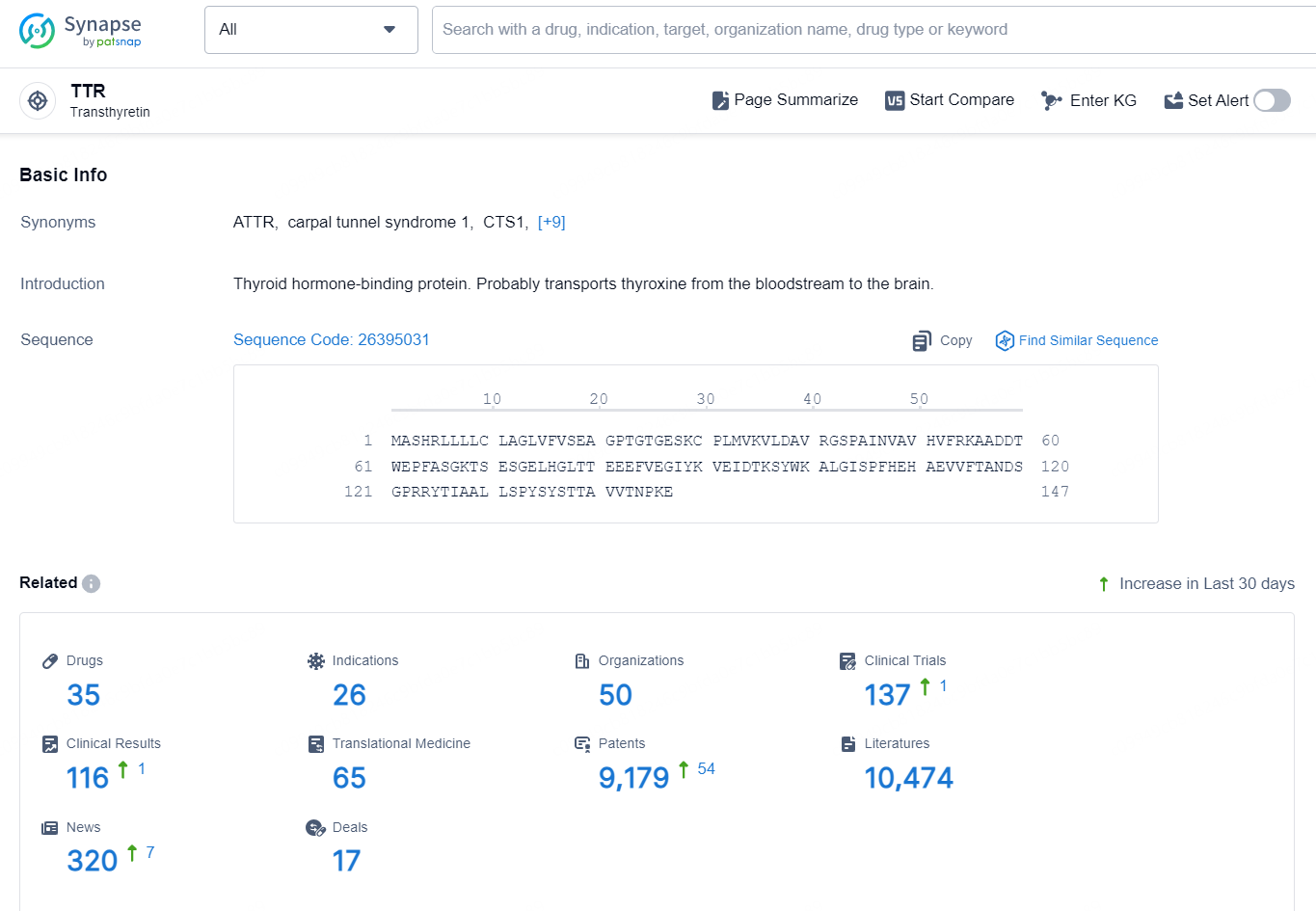
According to the data provided by the Synapse Database, As of October 18, 2024, there are 35 investigational drugs for the TTR targets, including 26 indications, 50 R&D institutions involved, with related clinical trials reaching 137, and as many as 9179 patents.
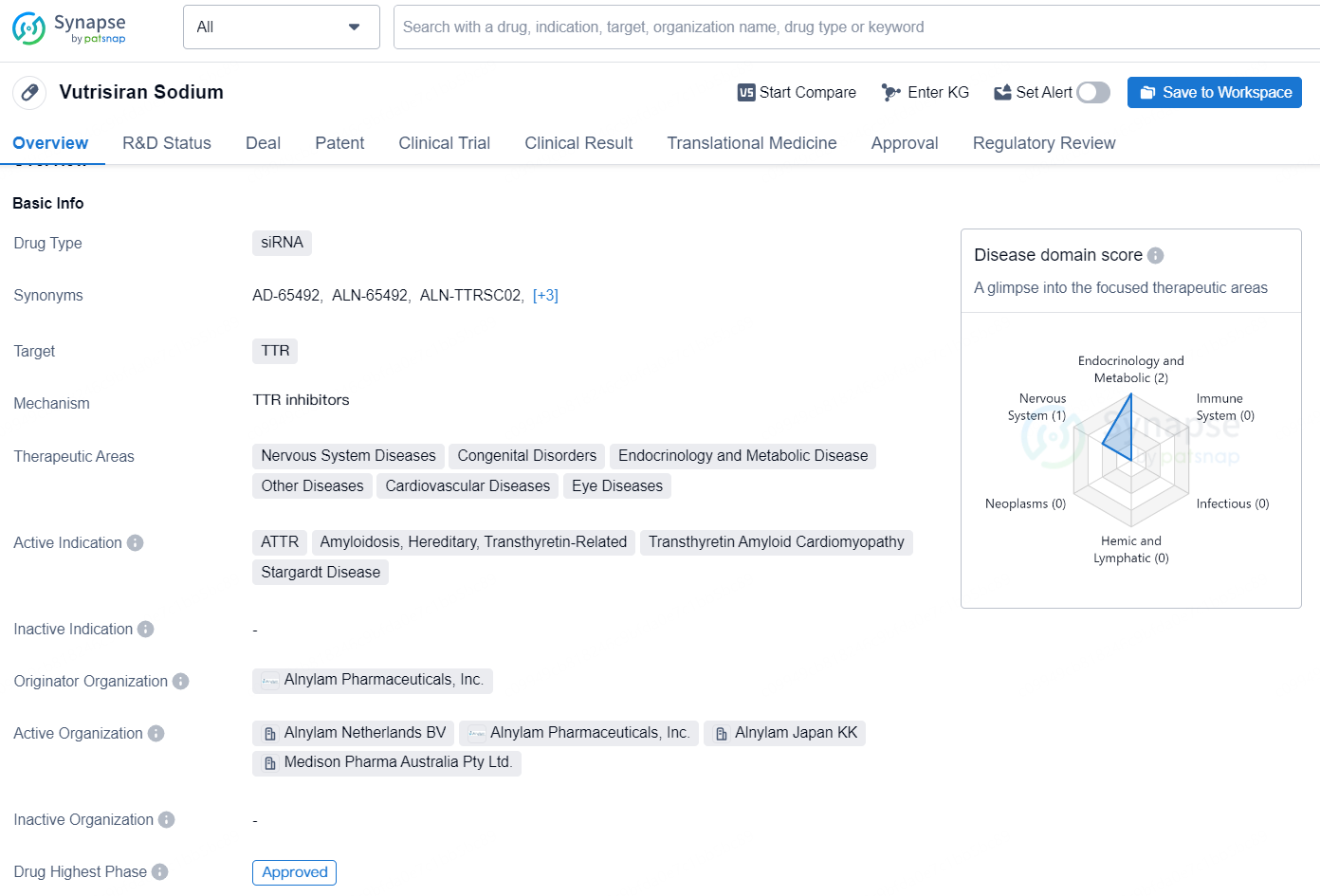
Vutrisiran Sodium is a drug belonging to the class of siRNA, which targets the TTR protein. Its therapeutic areas include Nervous System Diseases, Congenital Disorders, Endocrinology and Metabolic Disease, Other Diseases, Cardiovascular Diseases, and Eye Diseases. The drug is currently indicated for the treatment of ATTR (Transthyretin Amyloidosis), Hereditary Amyloidosis, Transthyretin-Related Amyloidosis, Transthyretin Amyloid Cardiomyopathy, and Stargardt Disease.