Dostarlimab gxly - The 4th Pan-Tumor Anti-Cancer Drug
Dostarlimab gxly, trade name JEMPERLI, is a monoclonal antibody targeting PD-1, originally discovered by AnaptysBio, Inc., and signed a cooperation and exclusive license agreement with TESARO, Inc. (a GSK subsidiary) in March 2014. This product was first approved in the European Union on April 21, 2021, for the treatment of adult patients with mismatch repair defects (dMMR)/microsatellite high instability (MSI-H) recurrence or advanced endometrial cancer (EC) . On June 6, 2023, the FDA approved inclusion in the priority review procedure for the first-line treatment of dMMR/MSI-H primary recurrence or advanced EC with Dostarlimab gxly combined with chemotherapy.

According to “Synapse”, this drug has a total of 21 indications under study, and all the indications, such as Mismatch repair-deficient Solid Tumor, sMismatch repair-deficient Endometrial Carcinoma, Endometrial Carcinoma, Microsatellite instability-high Endometrial Carcinoma, Colonic Cancer, Non-Small Cell Lung Cancer, et al and 68 clinical projects have been carried out. Single drug treatment for dMMR recurrence or advanced EC and solid tumors has been approved by FDA. For more detailed information on R&D status, core patents, analysis, etc., please click on the image link below.
This drug has obtained many regulations in different countries/regions, such as Breakthrough Therapy(US).

Mechanism of Action
Dostarlimab gxly is an IgG4 homologous humanized monoclonal antibody that binds to the PD-1 receptor, blocks its interaction with PD-L1 and PD-L2, releases PD-1 pathway mediated immune suppression, and promotes anti-tumor immune response.
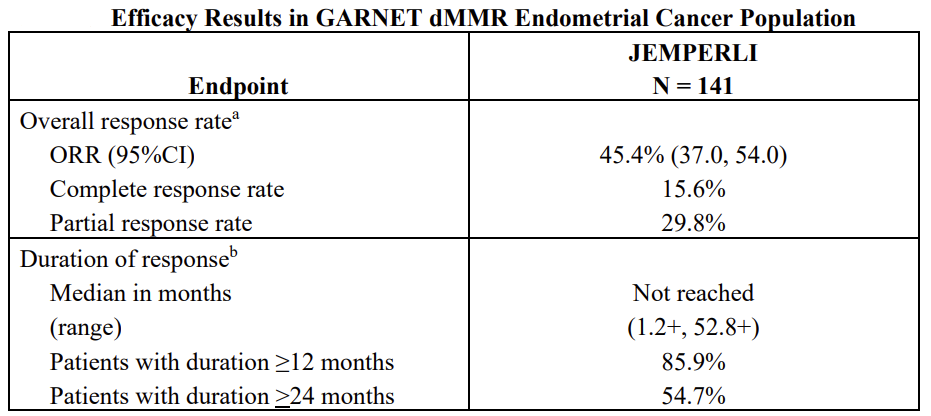
Efficacy: Monotherapy[13] : The efficacy of JEMPERLI was evaluated in the GARNET trial (NCT02715284), a multicenter, multicohort, open-label trial conducted in patients with advanced solid tumors. Efficacy results are presented below, which lays the foundation for Combination therapy.
Combination therapy
In a phase 3, global, double-blind, randomized, placebo-controlled trial, eligible patients with primary advanced stage III or IV or first recurrent EC were randomly assigned in a 1:1 ratio to receive either dostarlimab (500 mg) or placebo, plus carboplatin and paclitaxel, every 3 weeks (six cycles), followed by dostarlimab (1000 mg) or placebo every 6 weeks for up to 3 years. The primary end points were progression-free survival as assessed by the investigator according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, and overall survival. Results: In the dMMR–MSI-H population, estimated progression-free survival at 24 months was 61.4% in the dostarlimab group and 15.7% in the placebo group. In the overall population, progression-free survival at 24 months was 36.1% in the dostarlimab group and 18.1% in the placebo group. Overall survival at 24 months was 71.3% with dostarlimab and 56.0% with placebo.The most common adverse events that occurred or worsened during treatment were nausea, alopecia, and fatigue. Severe and serious adverse events were more frequent in the dostarlimab group than in the placebo group.

Competitive Landscape
Competitive products with the same indications: EC is the most common gynecological malignancy in developed countries, with approximately 60,000 new cases diagnosed in the US each year. An estimated 15-20% of EC patients are diagnosed at an advanced stage at the time of diagnosis.It is estimated that 20-29% of all ECs are dMMR/MSI-H. Monotherapy with chemotherapy is currently the standard treatment for primary advanced or recurrent EC, which is also the main competitive therapy for Dostarlimab-gxly plus chemotherapy. Competitive products with the same target: According to data from the Synapse, there are 301 PD-1 targeted drugs, of which 16 have been approved for marketing. Competition in the PD-1 field is intense.