DP Tech Unveils Kv1.3 Blocker Candidate for IBD and AD Treatment
DP Technology, a firm guided by the innovative approach of "AI for Science," has declared the selection of DPT0218 as a new preclinical candidate. This cutting-edge small molecule is designed to interact with Kv1.3 and shows promise as a potential treatment option for a range of autoimmune conditions such as Inflammatory Bowel Disease and Atopic Dermatitis.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.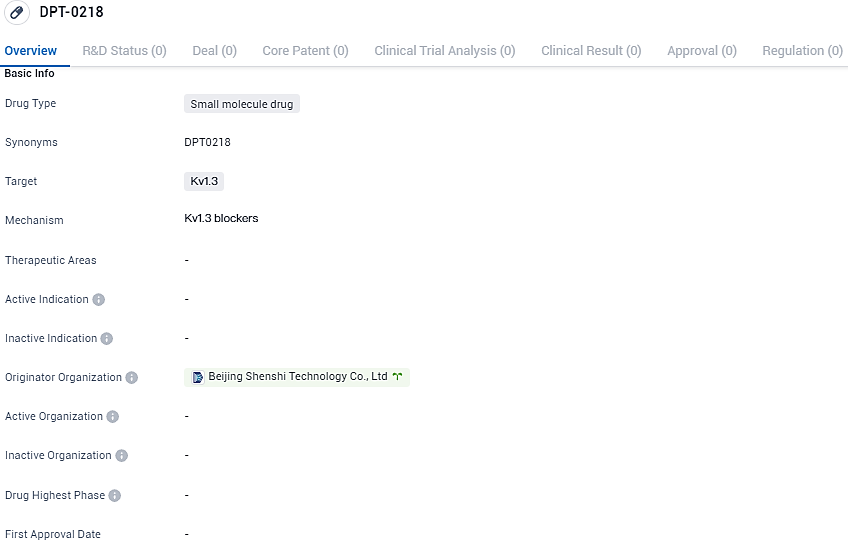
Kv1.3, a voltage-regulated potassium channel, plays a critical role in preserving the membrane potential necessary for initiating inflammatory actions; its obstruction diminishes immune cell activation and proliferation via calcineurin. Therefore, targeting Kv1.3 inhibition could play a significant role in tempering the activity and multiplication of inflammatory immune cells, such as T cells activated by antigens.
In a financial collaboration, Eli Lilly and Company provided an upfront sum of $60 million to D. E. Shaw Research for the development of DES-7114, the prominent candidate in the therapeutics program focusing on Kv1.3, with the possibility of additional payments that could reach $475 million linked to developmental and market success milestones.
The modulation of T cell activity has garnered attention as a promising target in the therapeutic landscape for IBD. Memory T cells, particularly those residing in tissues, are believed to perpetuate IBD, offering new avenues for treatment. Notably, an uptick in Kv1.3 channels was observed in PBMCs from patients with Ulcerative Colitis, showing a correlation with an increase in both Th1 and Th2 T cell populations. This elevated expression was also confirmed in T cells derived from UC patient intestinal biopsies.
The IBD patient demographic is significant, with at least 8 million affected globally, and over 5 million in Europe and North America. The autoimmune pharmaceutical market is anticipated to surge to $140 billion by 2028, with IBD treatments predicted to constitute $28 billion of this forecast, which equates to a 20% segment of the entire market.
A groundbreaking Kv1.3 antagonist, DPT0218, is recognized as potentially the top compound in its class with a pioneering structural framework. In preclinical trials using animal models, this agent has demonstrated promising outcomes against diverse autoimmune conditions, including IBD and Atopic Dermatitis. It boasts exceptionally potent activity at sub-nanomolar levels and showcases a remarkable 2000-fold specificity for the Kv1.3 channel compared to a wide spectrum of other ion channels such as Na+, K+, and Ca2+.
Additionally, DPT0218 features distinctive pharmacokinetics and pharmacodynamics, exhibiting concentrated drug levels in the colon and ileum yet maintaining minimal systemic presence across various species. Preliminary safety evaluations in these models indicate a robust safety profile.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!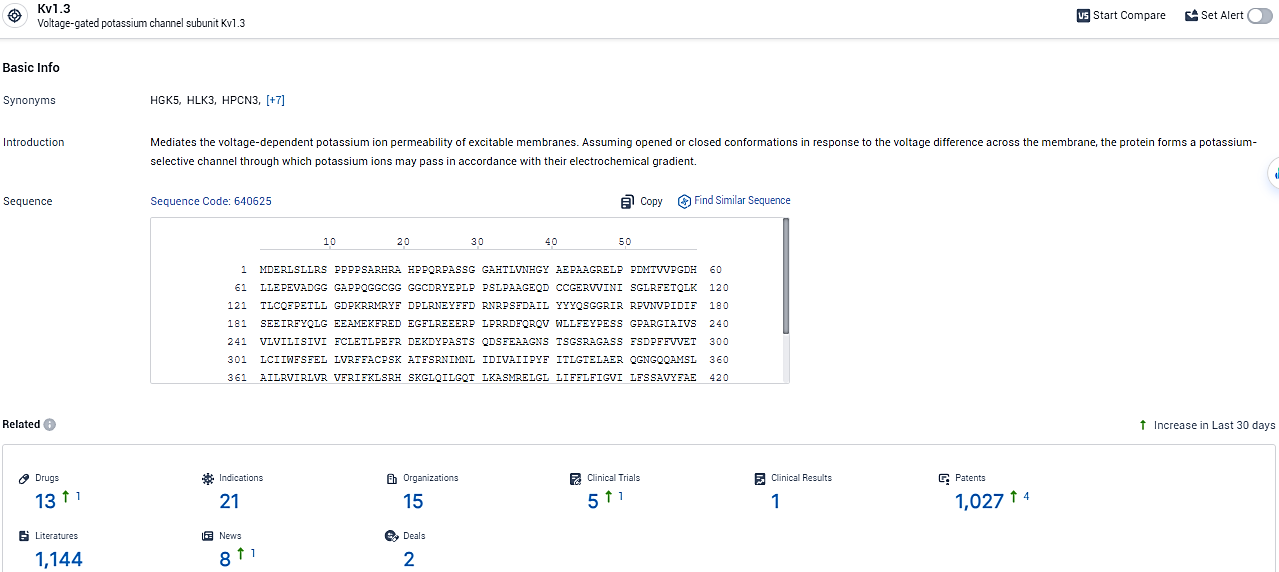
According to the data provided by the Synapse Database, As of February 6, 2024, there are 13 investigational drugs for the Kv1.3 target, including 21 indications, 15 R&D institutions involved, with related clinical trials reaching 5, and as many as 1027 patents.
With its potential to modulate T cell activity, DPT-0218 holds promise for the treatment of immune-related diseases. The drug's originator organization, known for its expertise in biomedicine, is dedicated to advancing therapeutic options and bringing innovative pharmaceutical solutions.




