Is Lurbinectedin approved by the FDA?
Yes, lurbinectedin (brand name: Zepzelca) is FDA approved. The U.S. Food and Drug Administration (FDA) granted accelerated approval to lurbinectedin on June 15, 2020.
What is Lurbinectedin?
Lurbinectedin is an intravenous medication used to treat small cell lung cancer (SCLC) that has metastasized, or spread to other parts of the body. It is specifically used in patients whose disease has progressed after receiving platinum-based chemotherapy.
How Does Lurbinectedin Work?
Lurbinectedin belongs to the drug class known as alkylating agents. It works by binding to the DNA in cancer cells, interfering with their ability to replicate and ultimately leading to cell death. This mechanism helps to slow down or stop the progression of cancer.
Usage and Administration
Lurbinectedin is administered as an intravenous infusion. The recommended dosage is 3.2 mg/m², given over 60 minutes every 21 days. Before administration, a blood test is often conducted to check liver function and ensure that the patient's absolute neutrophil count (ANC) and platelet count are sufficient for treatment.
Pre-treatment and Monitoring:
- Patients may receive pre-infusion medications such as corticosteroids and serotonin antagonists to prevent nausea and vomiting.
- Regular blood tests are necessary to monitor blood cell counts and assess the patient's response to treatment.
Precautions and Considerations
Before Treatment:
- Inform your doctor if you have liver or kidney disease.
- Women should use effective birth control during treatment and for at least six months after the last dose to prevent pregnancy. Men should use birth control if their partner is capable of becoming pregnant, continuing for at least four months after the last dose.
- Breastfeeding should be avoided during treatment and for at least two weeks after the last dose.
Side Effects
Common Side Effects:
- Low blood cell counts (anemia, leukopenia)
- Nausea, vomiting, loss of appetite
- Diarrhea or constipation
- Muscle or joint pain
- Fatigue
- Abnormal blood tests
Serious Side Effects:
- Easy bruising, unusual bleeding
- Severe anemia (pale skin, unusual tiredness)
- Low white blood cell counts (fever, mouth sores, cough)
- Signs of sepsis (confusion, severe drowsiness, rapid breathing)
- Liver problems (loss of appetite, upper right side stomach pain, jaundice)
Patients experiencing any serious side effects should contact their healthcare provider immediately.
Conclusion
Lurbinectedin, marketed as Zepzelca, is an FDA-approved treatment for metastatic small cell lung cancer, providing a valuable option for patients whose cancer has progressed after initial chemotherapy. Approved on June 15, 2020, lurbinectedin offers hope for managing and slowing the progression of this aggressive cancer.
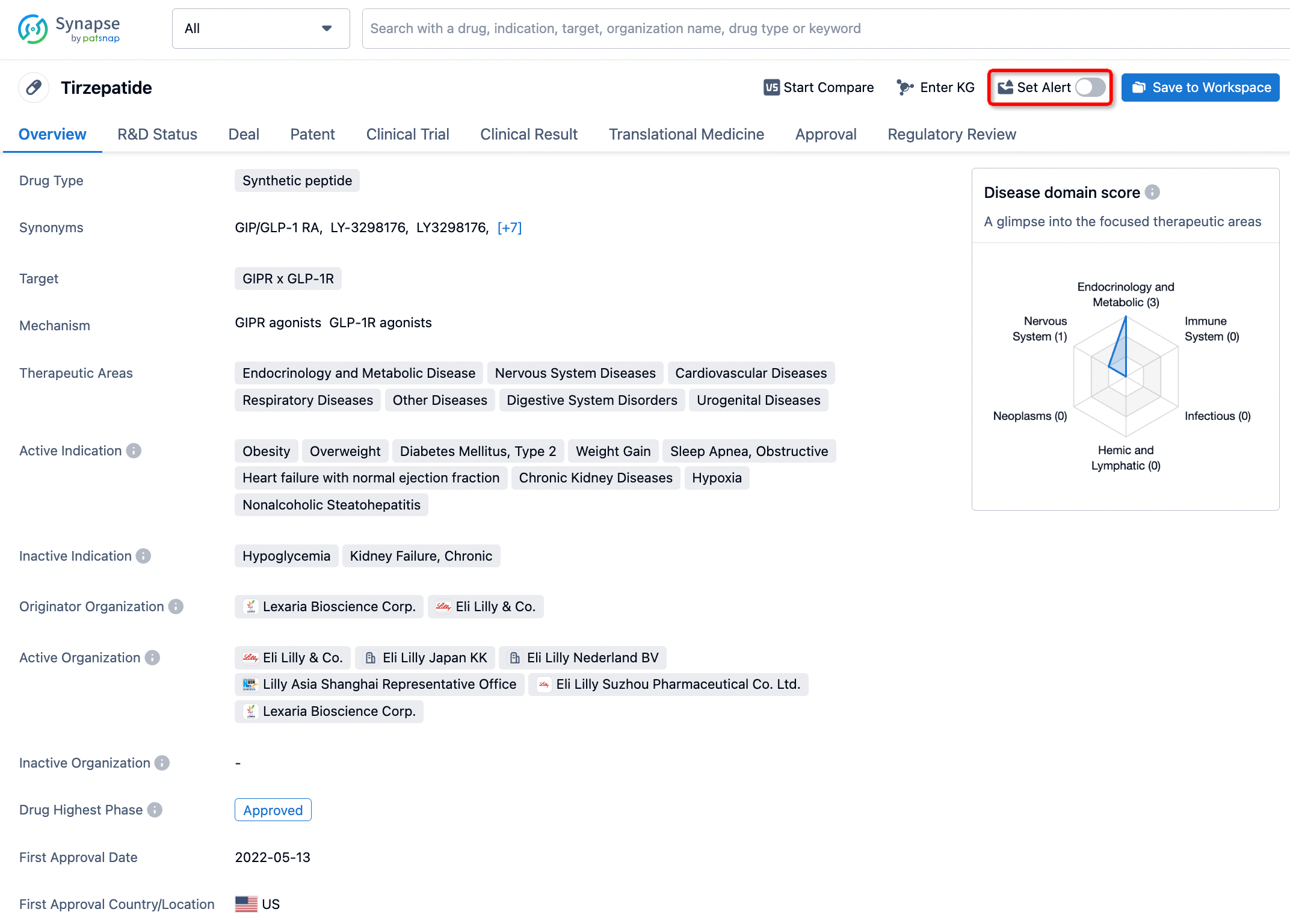
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!