Elevation Oncology's Phase 1 Trial: Promising Early Results for EO-3021 in Advanced Non-Resectable/Metastatic Solid Tumors
Elevation Oncology, Inc., a cutting-edge company specializing in oncology and dedicated to the discovery and development of targeted cancer therapies for patients suffering from various solid tumors with considerable unmet medical needs, has revealed encouraging preliminary data from the dose escalation segment of its ongoing Phase 1 clinical trial of EO-3021. The trial involves patients with advanced, inoperable, or metastatic solid tumors that are likely to express Claudin 18.2, such as gastric, gastroesophageal junction, pancreatic, or esophageal cancers.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
“Gastric and GEJ cancers represent severe diseases, both in the U.S. and globally, marked by high mortality rates despite recent medical advancements. There is an urgent demand for highly selective treatments for patients with Claudin 18.2-expressing tumors,” noted Kohei Shitara, M.D., the Chief of the Department of Gastrointestinal Oncology at the National Cancer Center Hospital East in Kashiwa, Japan, and principal investigator of the Phase 1 clinical study.
"In light of this, I am enthusiastic about the preliminary data on EO-3021, which indicates it has the potential to transform the treatment paradigm for numerous patients suffering from gastric or GEJ cancer. I look forward to further investigating EO-3021 in the expansion phase of this Phase 1 clinical trial," he added.
“We are delighted to release initial data from our Phase 1 clinical trial of EO-3021. Designed for maximum efficacy with minimized risk of free MMAE, EO-3021 aims to provide patients with a safer treatment profile and offer physicians a more easily combinable therapeutic option. It is promising to see EO-3021’s site-specific conjugation effectively translate into clinical benefits, with minimal MMAE-linked toxicities reported during our Phase 1 trial,” stated Valerie Malyvanh Jansen, M.D., Ph.D., Chief Medical Officer of Elevation Oncology.
“Alongside the encouraging anti-tumor activity observed in patients with gastric or GEJ cancer, the data suggest that EO-3021 could be a leading Claudin 18.2 antibody drug conjugate. We are eager to move forward into the monotherapy dose expansion phase and begin our combination cohorts in the coming months, and share further trial data in the first half of 2025,” Malyvanh Jansen continued.
During the dose escalation phase of a Phase 1 clinical trial, EO-3021 was tested on patients with advanced, unresectable, or metastatic solid tumors likely to express Claudin 18.2, including those with gastric, GEJ, pancreatic, or esophageal cancers.
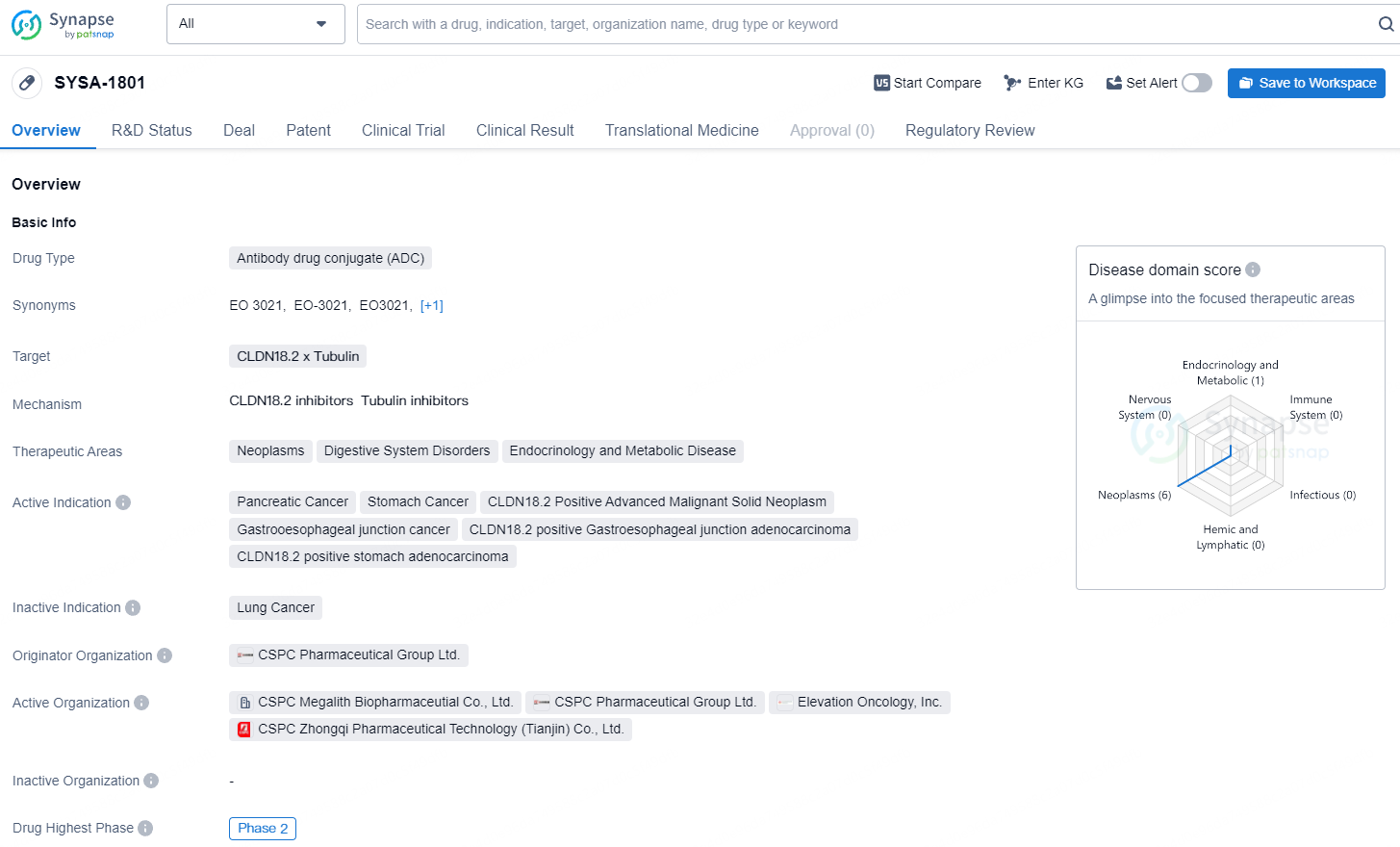
EO-3021 (also known as SYSA1801) stands out as a clinical-stage antibody drug conjugate with potential best-in-class status. It consists of an immunoglobulin G1 monoclonal antibody targeting Claudin 18.2, site-specifically conjugated to the monomethyl auristatin E payload via a cleavable linker, maintaining a drug-to-antibody ratio of 2.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
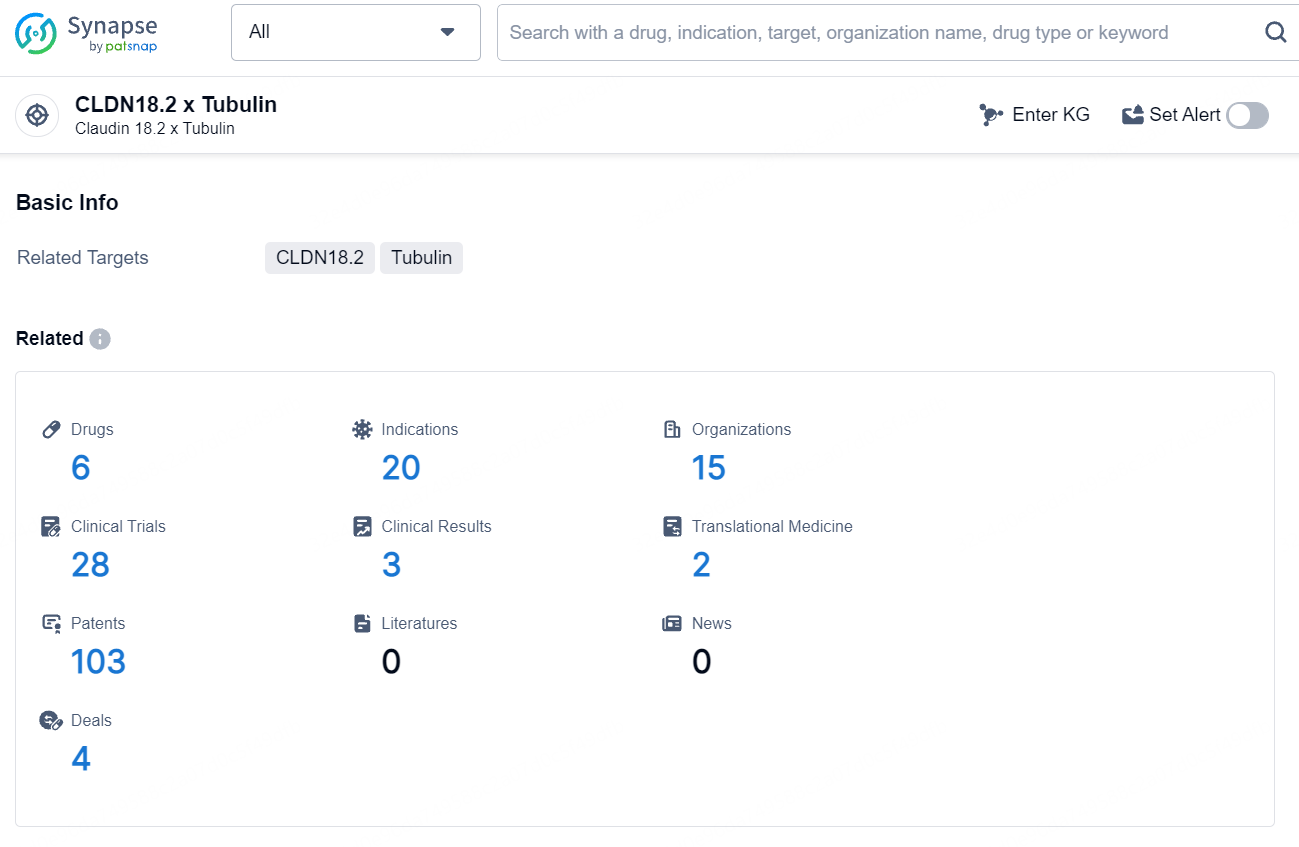
According to the data provided by the Synapse Database, As of August 9, 2024, there are 6 investigational drugs for the CLDN18.2 and Tubulin target, including 20 indications, 15 R&D institutions involved, with related clinical trials reaching 28, and as many as 103 patents.
SYSA-1801 has the potential to be a promising treatment option for various types of cancers, particularly those involving CLDN18.2 and Tubulin pathways. Further clinical development and regulatory evaluation will be crucial in determining the eventual impact and availability of SYSA-1801 in the pharmaceutical market.