Daiichi Sankyo and Merck Sign Global Pact for MK-6070 Development and Sales
Daiichi Sankyo and Merck, which operates as MSD in regions outside the U.S. and Canada, have broadened their current worldwide partnership on the joint development and commercialization of three experimental DXd antibody-drug conjugates. This expansion now encompasses Merck’s investigational product MK-6070, a T-cell engager targeting delta-like ligand 3 (DLL3).
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
 The enterprises will collaborate on the global development and marketing of MK-6070, with the exception of Japan, where Merck will retain exclusive rights. Merck will handle all manufacturing and supply responsibilities for MK-6070.
The enterprises will collaborate on the global development and marketing of MK-6070, with the exception of Japan, where Merck will retain exclusive rights. Merck will handle all manufacturing and supply responsibilities for MK-6070.
MK-6070 is a T-cell engager targeting DLL3, an inhibitory canonical Notch ligand highly expressed in small cell lung cancer and neuroendocrine tumors, currently under investigation in a phase 1/2 clinical trial.
Plans are underway to test MK-6070 in combination with ifinatamab deruxtecan (I-DXd) in specific patients with SCLC, alongside exploring other potential combinations. Merck acquired MK-6070 through its purchase of Harpoon Therapeutics.
“Enhancing our oncology pipeline with a DLL3 T-cell engager aligns with Daiichi Sankyo's strategy to set new standards of care for cancer patients globally,” stated Ken Takeshita, MD, Global Head of R&D at Daiichi Sankyo.
“We are eager to continue our partnership with Merck with the integration of MK-6070, which complements our existing antibody-drug conjugate collaboration, especially with ifinatamab deruxtecan, and underscores our mutual dedication to advancing innovative treatments for patients,” Ken Takeshita added.
“Small cell lung cancer is a highly aggressive and rapidly progressing lung cancer variant, necessitating novel treatment strategies,” commented Dean Y. Li, MD, PhD, President of Merck Research Laboratories. “We are excited to strengthen our collaboration with Daiichi Sankyo and anticipate evaluating the combined effects of MK-6070 and ifinatamab deruxtecan as a groundbreaking dual approach targeting the core biology of small cell lung cancer and other cancers.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
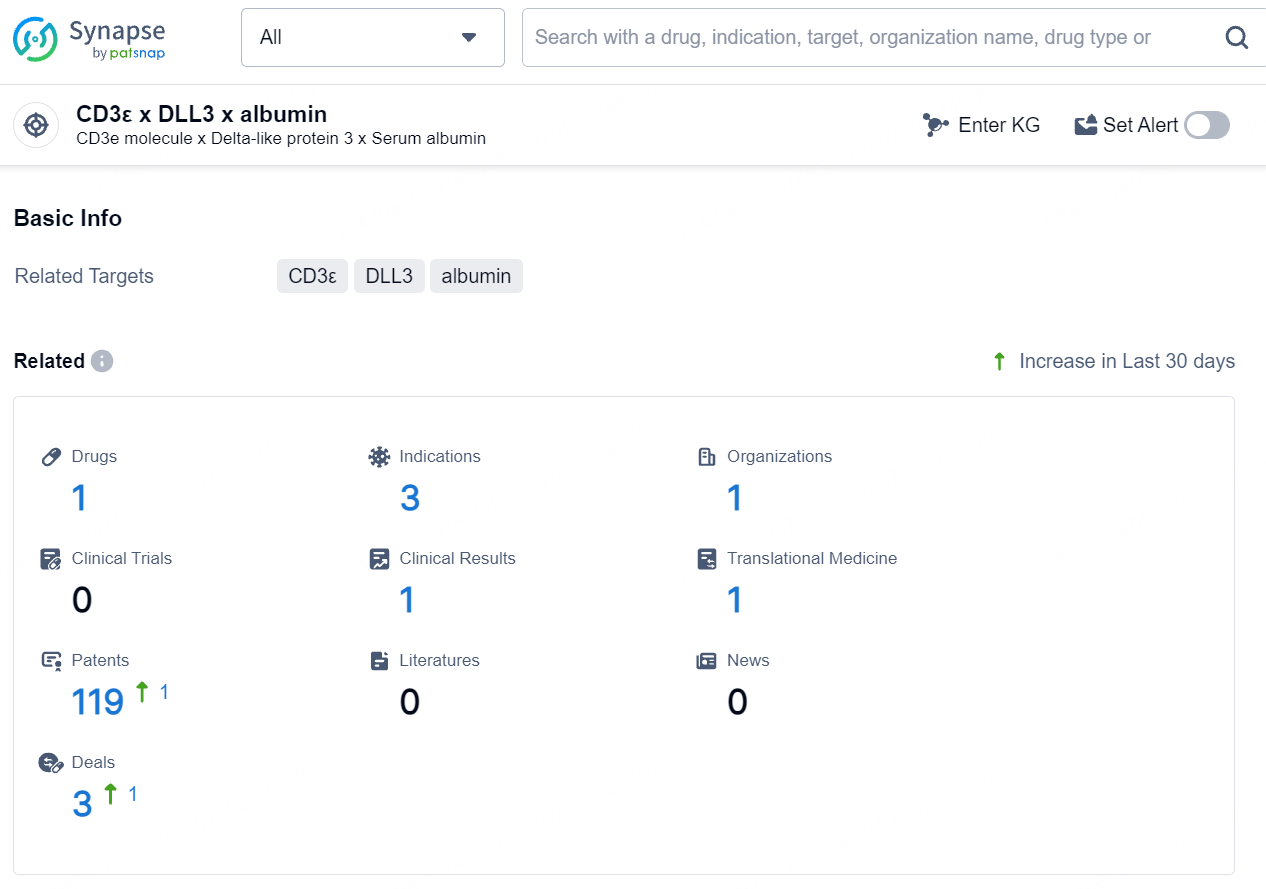
According to the data provided by the Synapse Database, As of August 9, 2024, there are 1 investigational drug for the CD3ε/DLL3/albumin target, including 3 indications, 1 R&D institution involved, and as many as 119 patents.
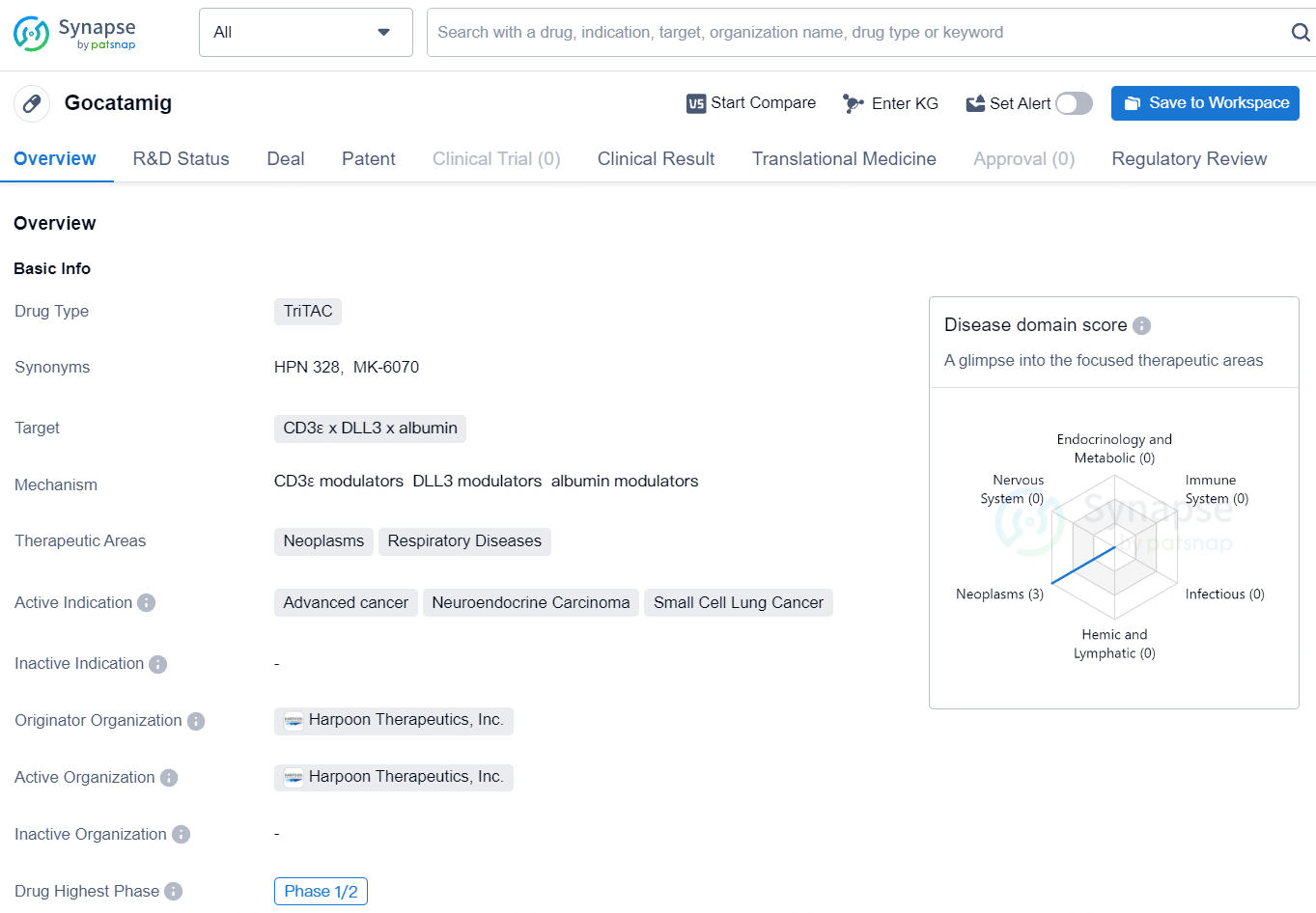
MK-6070 is an investigational DLL3 directed tri-specific T-cell engager currently being evaluated in a phase 1/2 clinical trial as a monotherapy in certain patients with advanced cancers associated with expression of DLL3 and in combination with atezolizumab in certain patients with SCLC. The U.S. Food and Drug Administration granted Orphan Drug Designation to MK-6070 for the treatment of SCLC in March 2022.