Lupin Introduces Rymti®, a Biosimilar of Etanercept, in Canada
Global pharma major Lupin Limited, revealed the introduction of its first biosimilar in Canada, Rymti®, via its collaborator, Sandoz Canada. Indications for Rymti® include moderate to severe active rheumatoid arthritis, juvenile idiopathic arthritis, active and progressive psoriatic arthritis, severe axial spondyloarthritis, moderate to severe plaque psoriasis, and chronic severe plaque psoriasis in pediatric patients. Rymti® has received approval for all therapeutic uses identical to its reference product, Enbrel.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
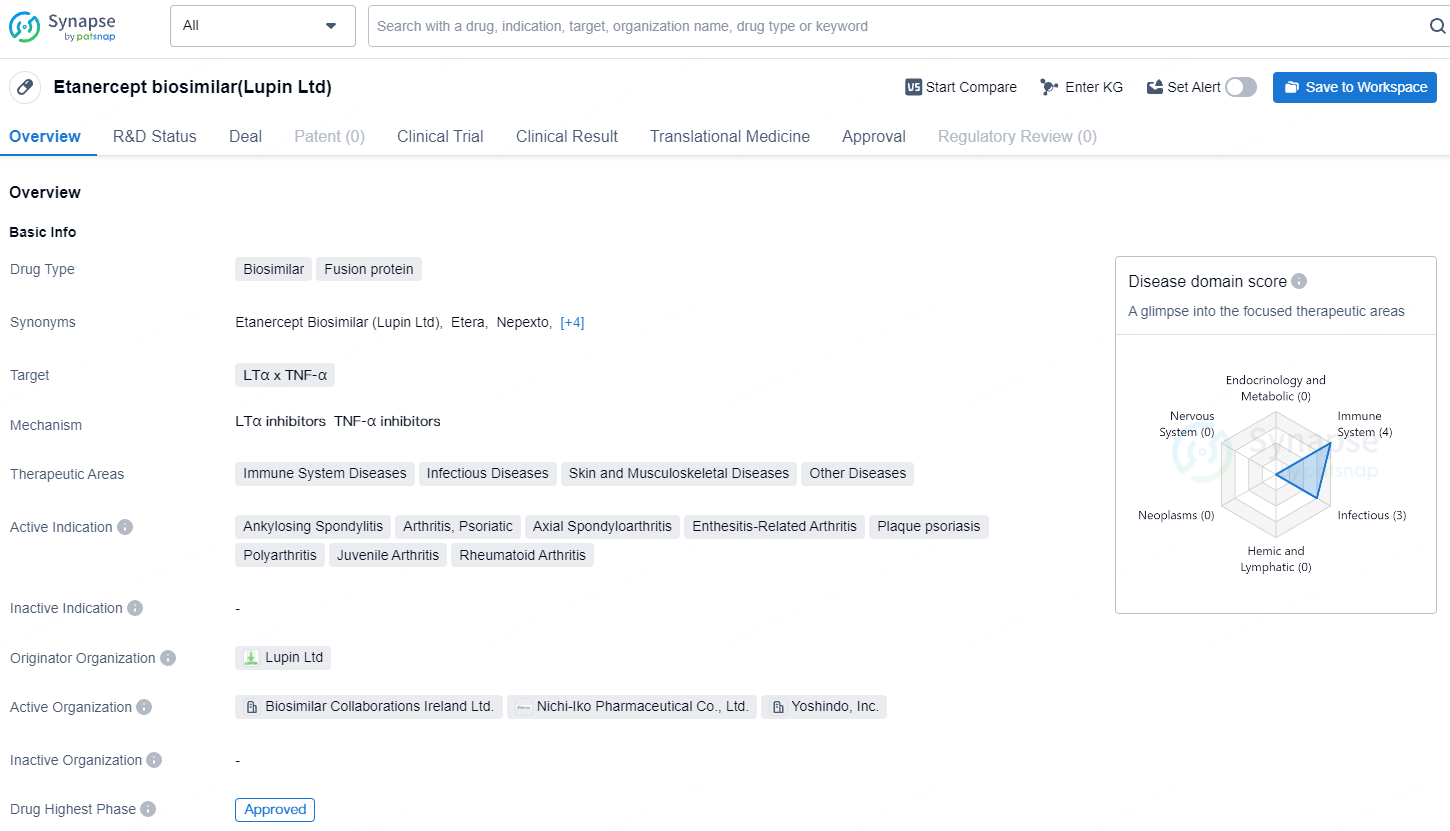
Lupin's Etanercept initially gained approval for the treatment of rheumatoid arthritis from PMDA in 2019, EMA in 2020, and Health Canada in 2022. Since then, it has provided an efficient solution for various chronic inflammatory conditions.
Rymti® comes as an injectable solution in both pre-filled pens and syringes. Studies indicate that patients highly accept the user-friendly pre-filled pen. This latex-free device is preferred by users for self-injection, which can enhance adherence to treatment.
Rymti® demonstrates equivalent efficacy and safety to the reference product Enbrel® and serves as a cost-effective treatment option that supports sustainable healthcare solutions. Lupin's etanercept biosimilar, Rymti, received Health Canada's approval in August 2022 for all the same indications as Enbrel.
"We're thrilled to introduce Rymti®, Lupin’s first biosimilar in Canada through our partner Sandoz," stated Vinita Gupta, Chief Executive Officer of Lupin. "This significant achievement helps us continue to enhance healthcare availability and affordability for Canadian patients."
"Etanercept is internationally utilized to combat multiple severe autoimmune diseases. We're excited to offer etanercept to patients in Canada. Our sustained efforts to expand our portfolio with etanercept ensure that patients have access to an effective and affordable alternative. This launch signifies our dedication to developing healthcare solutions that genuinely impact lives," remarked Dr. Cyrus Karkaria, President of Biotechnology at Lupin.
Michel Robidoux, President and General Manager of Sandoz Canada, commented, "Introducing Rymti® is a pivotal moment for Sandoz Canada. Our exclusive partnership with Lupin enables us to offer another etanercept option in the Canadian market, thereby increasing patient access."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
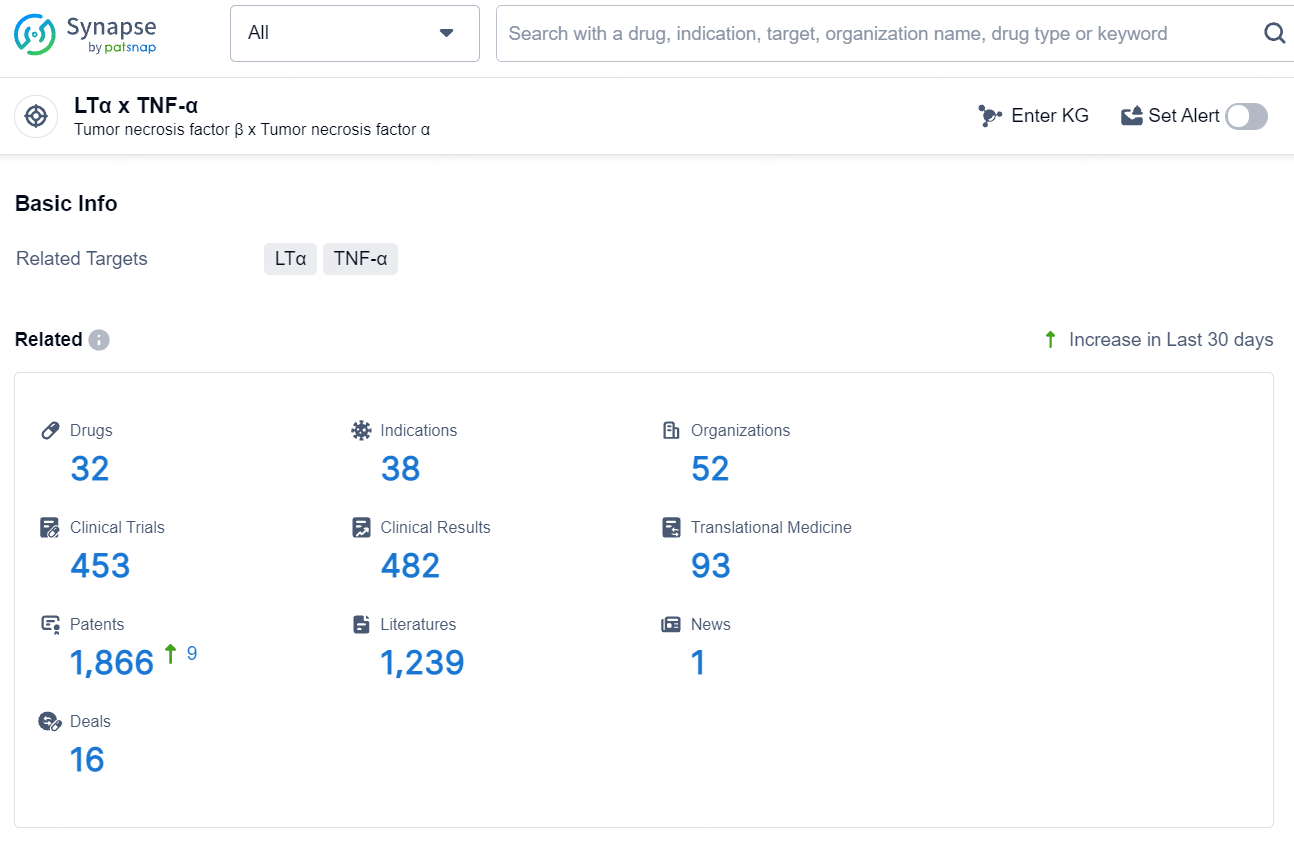
According to the data provided by the Synapse Database, As of August 9, 2024, there are 32 investigational drugs for the TNF and LTα target, including 38 indications, 52 R&D institutions involved, with related clinical trials reaching 453, and as many as 1866 patents.
Etanercept is an injectable biologic medicine that inhibits the biological activity of Tumor Necrosis Factor (TNF). TNF is a key cytokine involved in the pro-inflammatory cascade in many chronic, immune-mediated inflammatory diseases such as rheumatoid arthritis, psoriatic arthritis, axial spondyloarthritis and plaque psoriasis. Etanercept as a soluble TNF receptor fusion protein specifically binds to TNF and blocks its activity, thereby reducing inflammation and disease symptoms.