Lupin Announces the Successful Completion of Phase 3 Trials for Lucentis® Biosimilar
Global Pharma major Lupin Limited revealed the completion of an international Phase 3 clinical trial for its biosimilar LUBT010, which is an alternative to Lucentis. The trial has met its main goal by demonstrating therapeutic equivalence in enhancing visual acuity for patients with wet AMD, while also showing similar safety and immunogenicity profiles between LUBT010 and Lucentis.
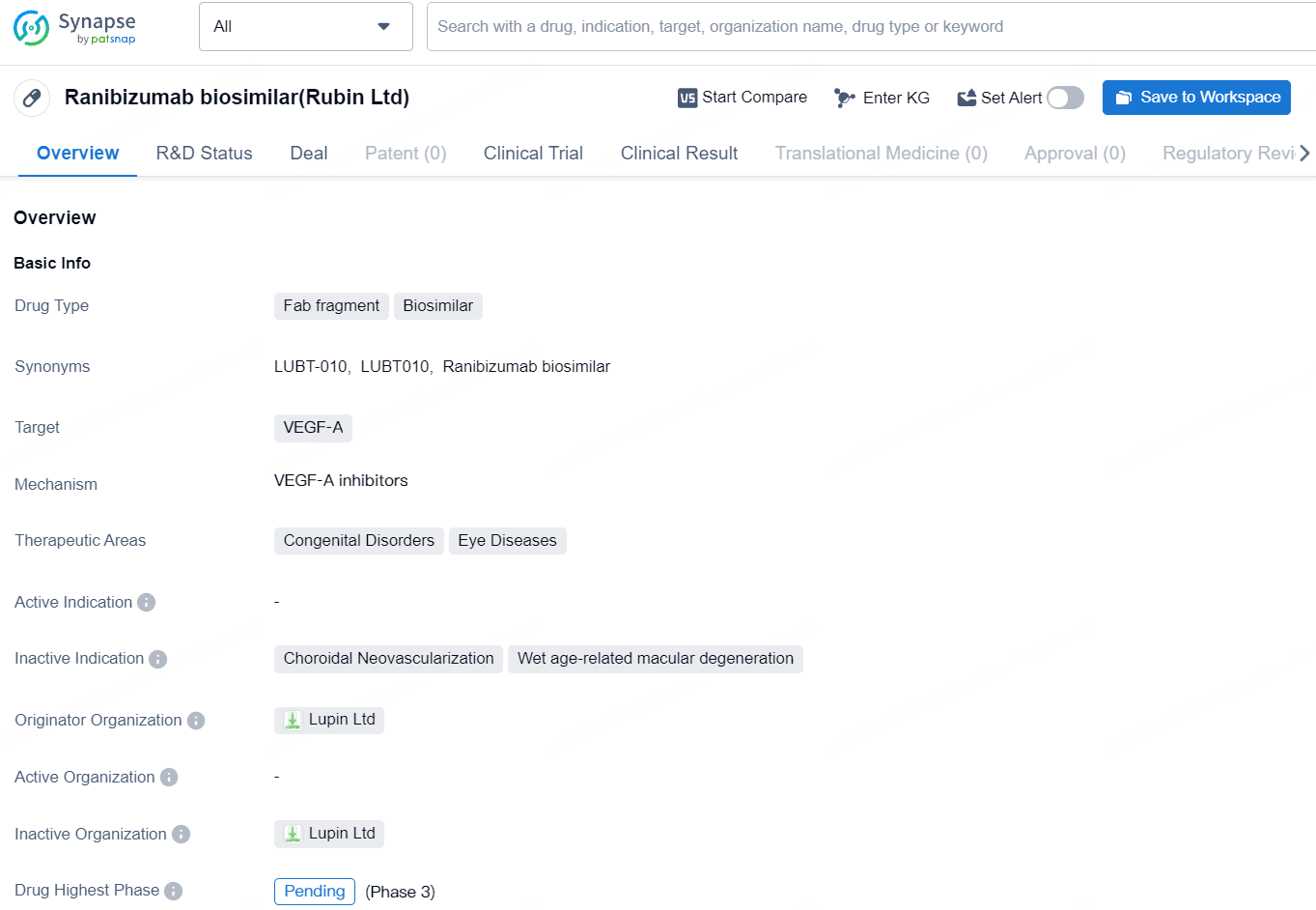
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Since 2022, Lupin has been marketing its ranibizumab biosimilar in India under the trade name RaniEyes. Ranibizumab, a recombinant humanized IgG1 kappa isotype monoclonal antibody fragment, is utilized in treating Neovascular Age-related Macular Degeneration, Macular Edema Following Retinal Vein Occlusion, Diabetic Macular Edema, Diabetic Retinopathy, and Myopic Choroidal Neovascularization.
The Phase 3 study was implemented as an international clinical trial, adhering to EMA and U.S. FDA guidelines, to assess the efficacy, safety, and immunogenicity of LUBT010 compared to Lucentis in patients with Neovascular Age-related Macular Degeneration. A cohort of 600 patients from India, the U.S., EU, and Russia was randomized to receive either LUBT010 or Lucentis® 0.5 mg, delivered via monthly intravitreal injections over a 12-month period.
Patients were monitored to evaluate efficacy, safety, and immunogenicity outcomes. The findings from this Phase 3 trial will be included in Lupin's regulatory submission for marketing approval with the U.S. FDA and the European Medicines Agency.
"We are extremely satisfied with the positive results of the global Phase 3 study, which signifies another crucial milestone in the development of our Lucentis® Biosimilar. This is a testament to our commitment to producing high-quality biosimilars that address patient needs," stated Dr. Cyrus Karkaria, President of Lupin Biotech. "We plan to file marketing applications for LUBT010 in all major global markets this year."
Reflecting on the achievement, Mr. Nilesh Gupta, Managing Director of Lupin, remarked, "This accomplishment of our Biosimilars team showcases our ability to develop advanced, cost-effective products. We have already commercialized four products and have several others at various clinical trial stages. We are excited to introduce our high-quality Ranibizumab Biosimilar into the global Ophthalmic market, positively impacting patients' lives worldwide."
Lupin, an innovation-driven multinational pharmaceutical company based in Mumbai, India, develops and markets a broad spectrum of branded and generic formulations, biotechnology products, and APIs across over 100 markets, including the U.S., India, South Africa, and regions throughout the Asia Pacific, Latin America, Europe, and the Middle East.
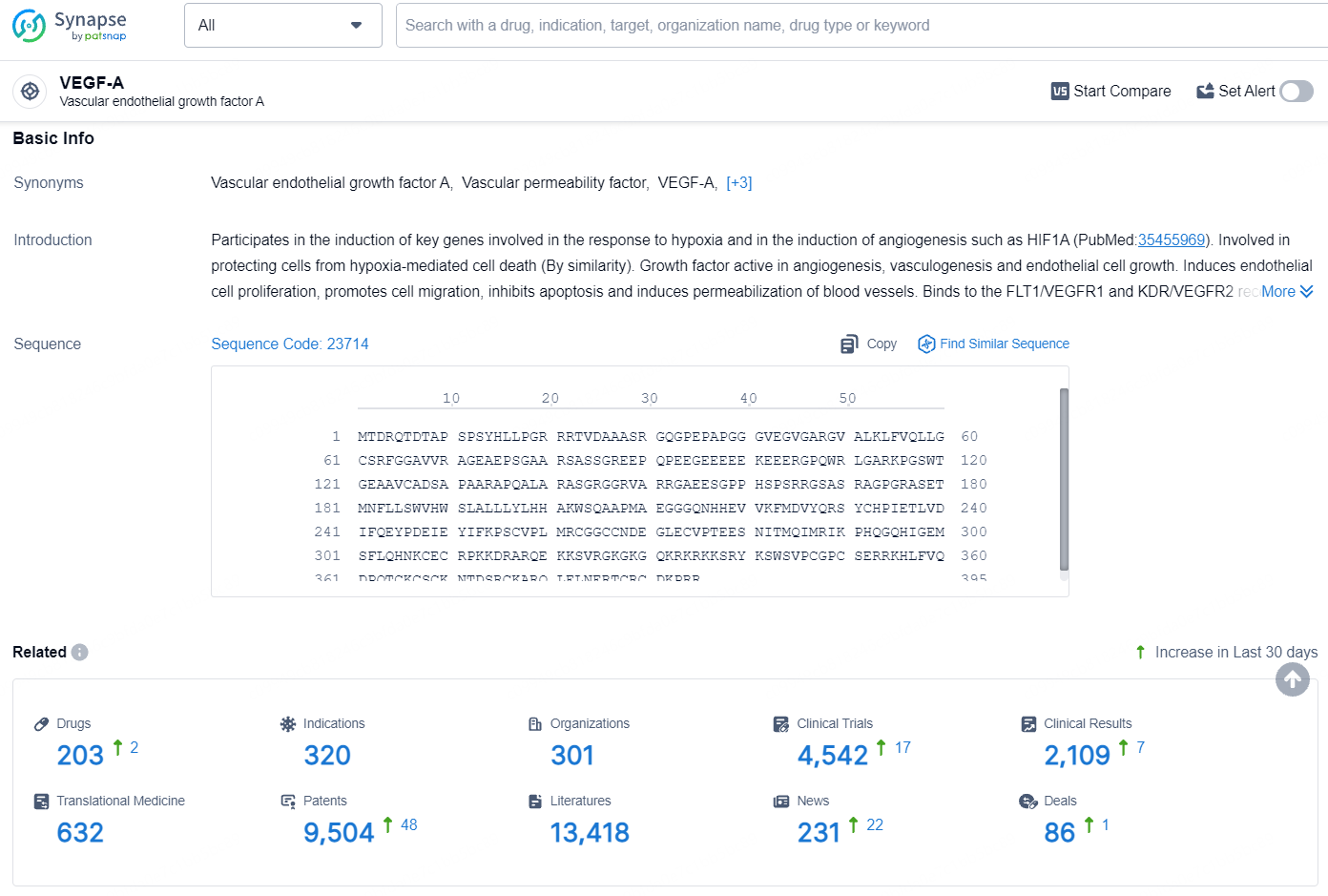
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 8, 2024, there are 203 investigational drugs for the VEGF-A targets, including 320 indications, 301 R&D institutions involved, with related clinical trials reaching 4542, and as many as 9504 patents.
LUBT010 is a Fab fragment biosimilar drug targeting VEGF-A for the treatment of congenital disorders and eye diseases. With its pending phase globally, this drug represents a potential advancement in the field of biomedicine for improving the management of eye-related conditions and supporting patients with congenital disorders.