Inmagene Activates Its Right to Secure Sole Global Licensing for IMG-007 and IMG-004 from HUTCHMED
Inmagene Biopharmaceuticals, an enterprise at the clinical phase specializing in the creation of unique therapeutic solutions for conditions related to immunity and inflammation, has declared the activation of its rights to acquire a solely controlled, global license involving royalties to IMG-007, a non-eliminating humanized antibody targeting OX40, and IMG-004, a systemically administered inhibitor of BTK that operates through non-covalent, reversible mechanisms. This license also encompasses the authority to sub-license these entities across various levels.
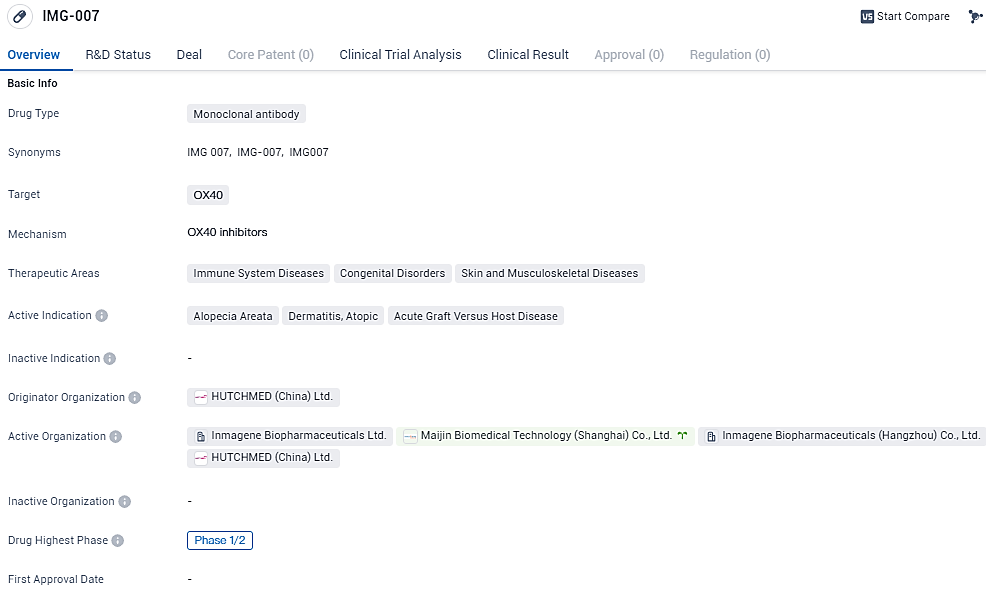
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Inmagene and HUTCHMED initiated a collaborative effort in 2021 aimed at advancing and commercializing a collection of investigational compounds targeting inflammatory and immunological (I&I) conditions. As a result of this cooperative initiative, the option was utilized.
"In our concerted efforts with HUTCHMED, we've managed to progress IMG-007 and IMG-004, which hold the promise of being at the forefront of their class, from the pre-clinical phase into the realm of clinical trials," stated Inmagene's Chief Executive Officer, Jonathan Wang, Ph.D. "Securing the global exclusive rights marks another milestone in our mission to achieving prominence in the innovative treatment discovery for I&I conditions."
Currently, IMG-007 is undergoing Phase 2a clinical trials for two specific disorders: severe forms of atopic dermatitis and alopecia areata. Meanwhile, IMG-004 is in the midst of a Phase 1 clinical trial to assess its safety and efficacy through multiple increasing doses.
The compound IMG-007 is a humanized monoclonal antibody (mAb) targeting the OX40 receptor (anti-OX40 IgG1), with a design focused on a prolonged half-life and an engineered reduction of antibody-dependent cell-mediated cytotoxicity (ADCC) functions. The interaction between the OX40 receptor and its ligand (OX40L) plays a critical part in activating and sustaining T cells, which is significant in the development of a wide range of I&I conditions. Preclinical investigations have shown that IMG-007 can effectively and selectively inhibit the OX40-OX40L signaling.
Early findings from Phase 1 single ascending dose (SAD) studies imply a prolonged half-life, approximately 31 days at doses that may have therapeutic effect, allowing for dosing every 12 weeks. Additionally, the safety profile appears favorable, distinct from other compounds under development, with no incidence of pyrexia or chills noted. These promising characteristics bolster the ongoing evaluation of IMG-007 for potential use in the treatment of severe types of atopic dermatitis and alopecia areata in two separate Phase 2a clinical trials.
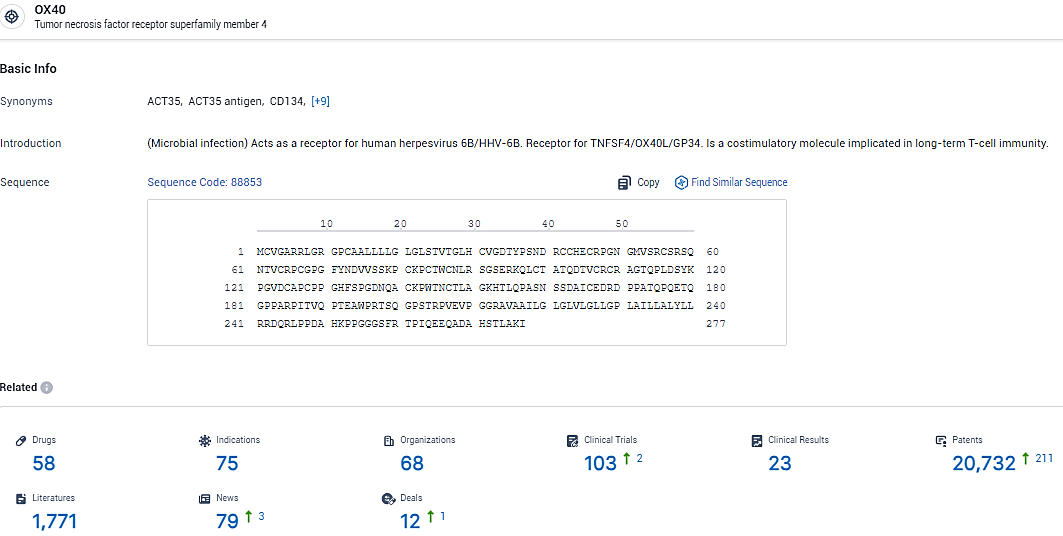
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 8, 2024, there are 58 investigational drugs for the OX40 target, including 75 indications, 68 R&D institutions involved, with related clinical trials reaching 103, and as many as 20732 patents.
IMG-007 targets OX40 and is being investigated for its potential in treating immune system diseases, congenital disorders, as well as skin and musculoskeletal diseases. Currently in Phase 1/2 of clinical development globally, IMG-007 has received IND approval in China.