EMA Approves AVT03, a Biosimilar to Prolia® and Xgeva®
Alvotech (NASDAQ: ALVO), a worldwide biotechnology firm focused on the creation and production of biosimilar pharmaceuticals for patients around the globe, revealed that the European Medicines Agency (EMA) has received a Marketing Authorization Application for AVT03, a proposed biosimilar to Prolia® and Xgeva® (denosumab).
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
“Receiving EMA acceptance represents a significant milestone in making AVT03 accessible to patients and their caregivers in Europe,” stated Joseph McClellan, Chief Scientific Officer of Alvotech. “The advancement in the development of various biosimilar candidates illustrates Alvotech's capacity to utilize its comprehensive biosimilars platform to promote wider access to cost-effective biologic therapies.”
Alvotech is responsible for the development and manufacturing of AVT03. STADA Arzneimittel AG and Dr. Reddy’s Laboratories SA have established partnerships with Alvotech for the marketing of AVT03, each holding semi-exclusive commercial rights across Europe, including the UK and Switzerland.
In July 2024, Alvotech revealed encouraging topline findings from a confirmatory study involving patients for AVT03. The AVT03-GL-C01 trial, which achieved its primary objectives, showed clinical similarity between AVT03 and Prolia regarding efficacy, safety, immunogenicity, and pharmacokinetics (PK) among 532 postmenopausal women suffering from osteoporosis. The primary objectives were also accomplished in both the AVT03-GL-P01 study, which evaluated the PK, safety, and tolerability of AVT03 against Prolia in 209 healthy adults, and the AVT03-GL-P03 study that explored the PK, safety, and tolerability of AVT03 in comparison to Xgeva in 208 healthy adult participants.
Prolia is approved for treating osteoporosis in postmenopausal women and men at a higher risk of fractures; it addresses bone loss in men undergoing prostate cancer treatments that elevate their fracture risk; as well as in adults at increased risk of fractures who are undergoing long-term therapy with oral or Injectable corticosteroids. Xgeva is indicated for preventing bone complications in adults with advanced cancer that has metastasized to the bone and for the treatment of giant cell tumor of bone in adults and fully developed adolescents.
The overall market for denosumab in Europe is currently estimated to be around US$1 billion. The introduction of biosimilar products for Prolia and Xgeva has the potential to greatly enhance patient access while maintaining or reducing overall costs.
In 2019, the estimated total direct costs of osteoporotic fractures in the European Union, Switzerland, and the UK amounted to US$63 billion, not accounting for the individual burden of the disease. Approximately 32 million people in Europe had osteoporosis, with 80% being women; however, 70% of women who were eligible for treatment did not receive care for their osteoporosis.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
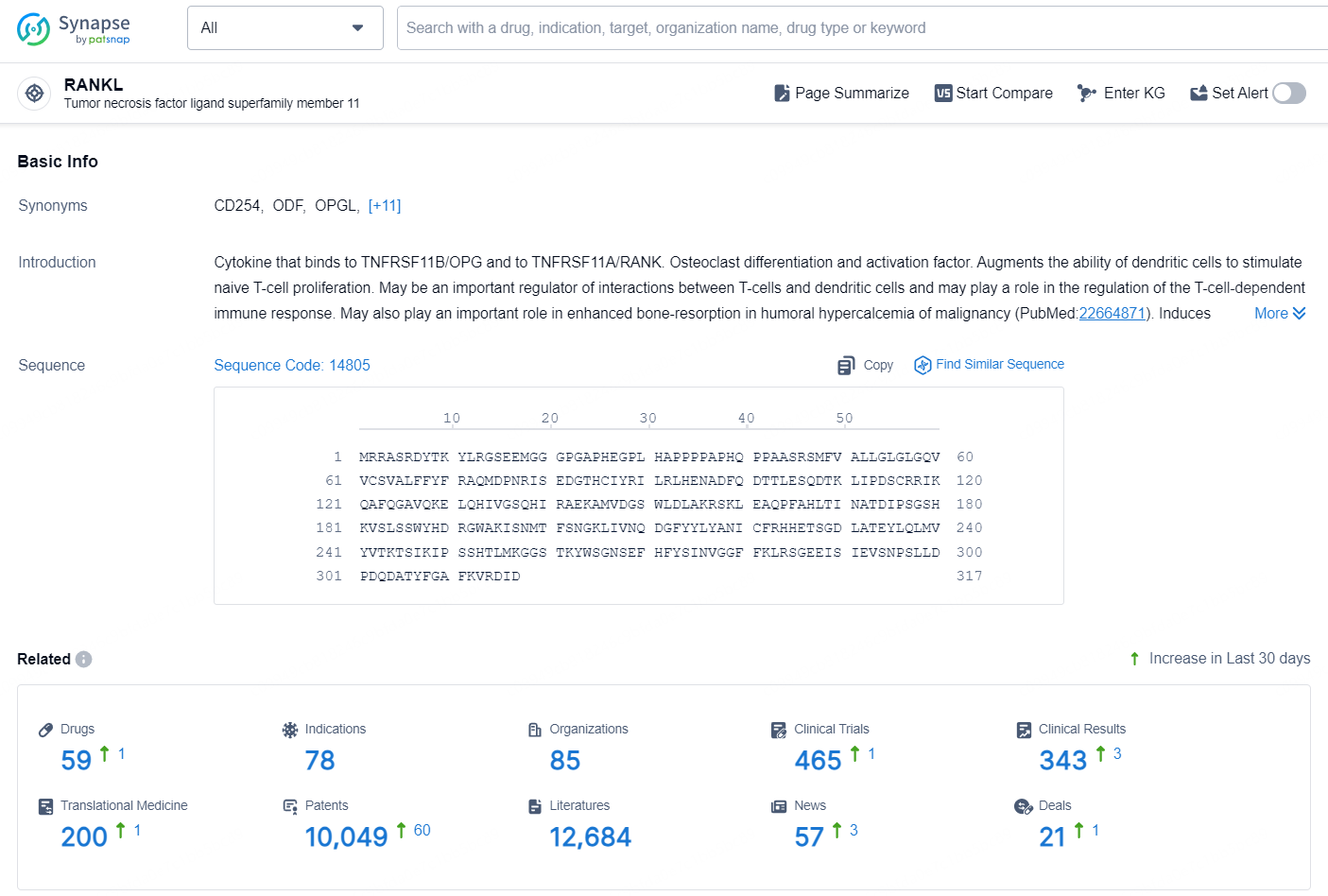
According to the data provided by the Synapse Chemical, As of October 15, 2024, there are 59 investigational drugs for the RANKL target, including 78 indications, 85 R&D institutions involved, with related clinical trials reaching 465, and as many as 10049 patents.
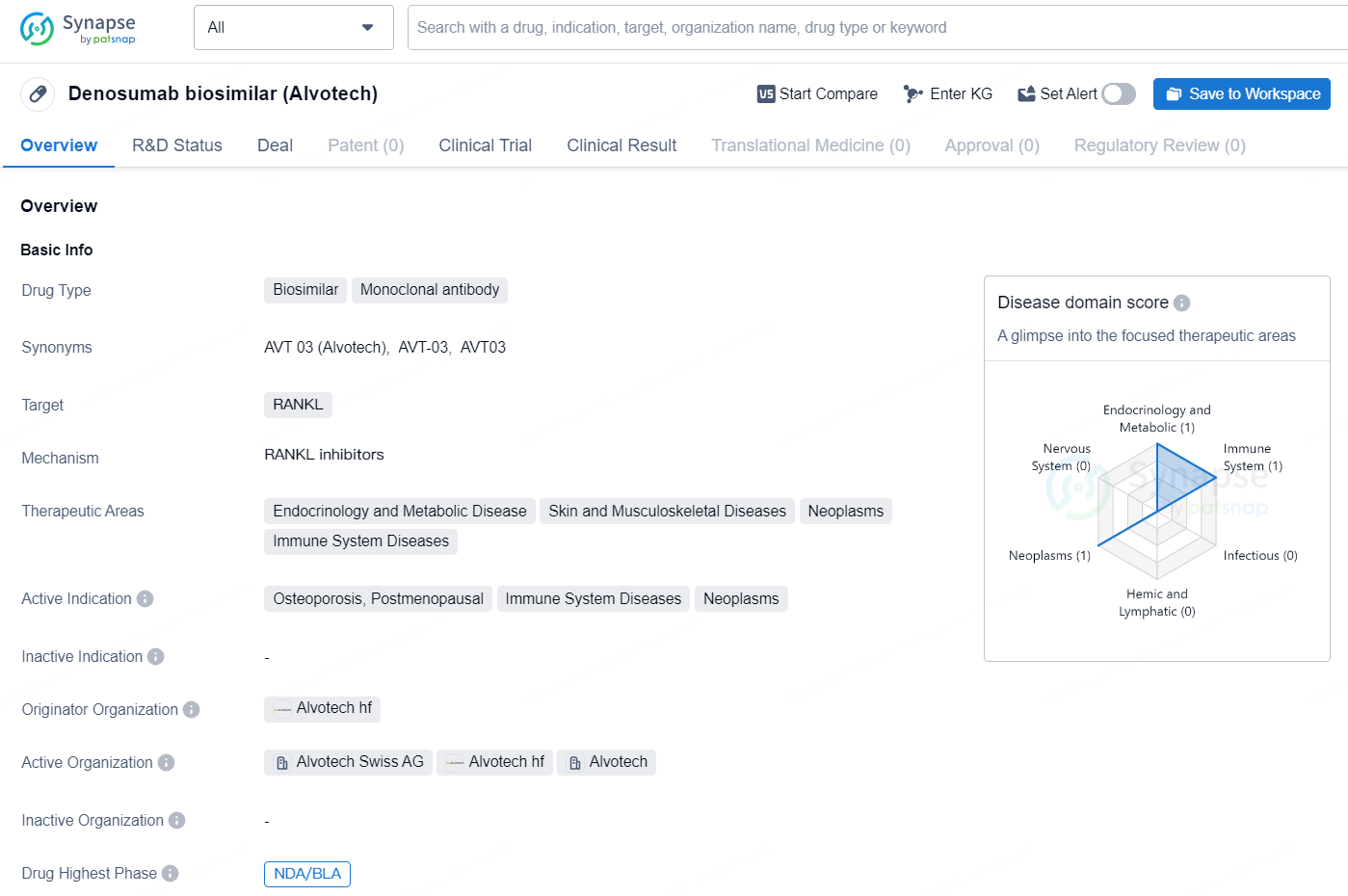
Denosumab biosimilar (Alvotech) is a biosimilar drug classified as a monoclonal antibody. It targets RANKL and is indicated for the treatment of various therapeutic areas including Endocrinology and Metabolic Disease, Skin and Musculoskeletal Diseases, Neoplasms, and Immune System Diseases. The active indications for this drug include Osteoporosis, Postmenopausal, Immune System Diseases, and Neoplasms.