Exploring Savolitinib's Revolutionary R&D Successes and its Mechanism of Action
Savolitinib's R&D Progress
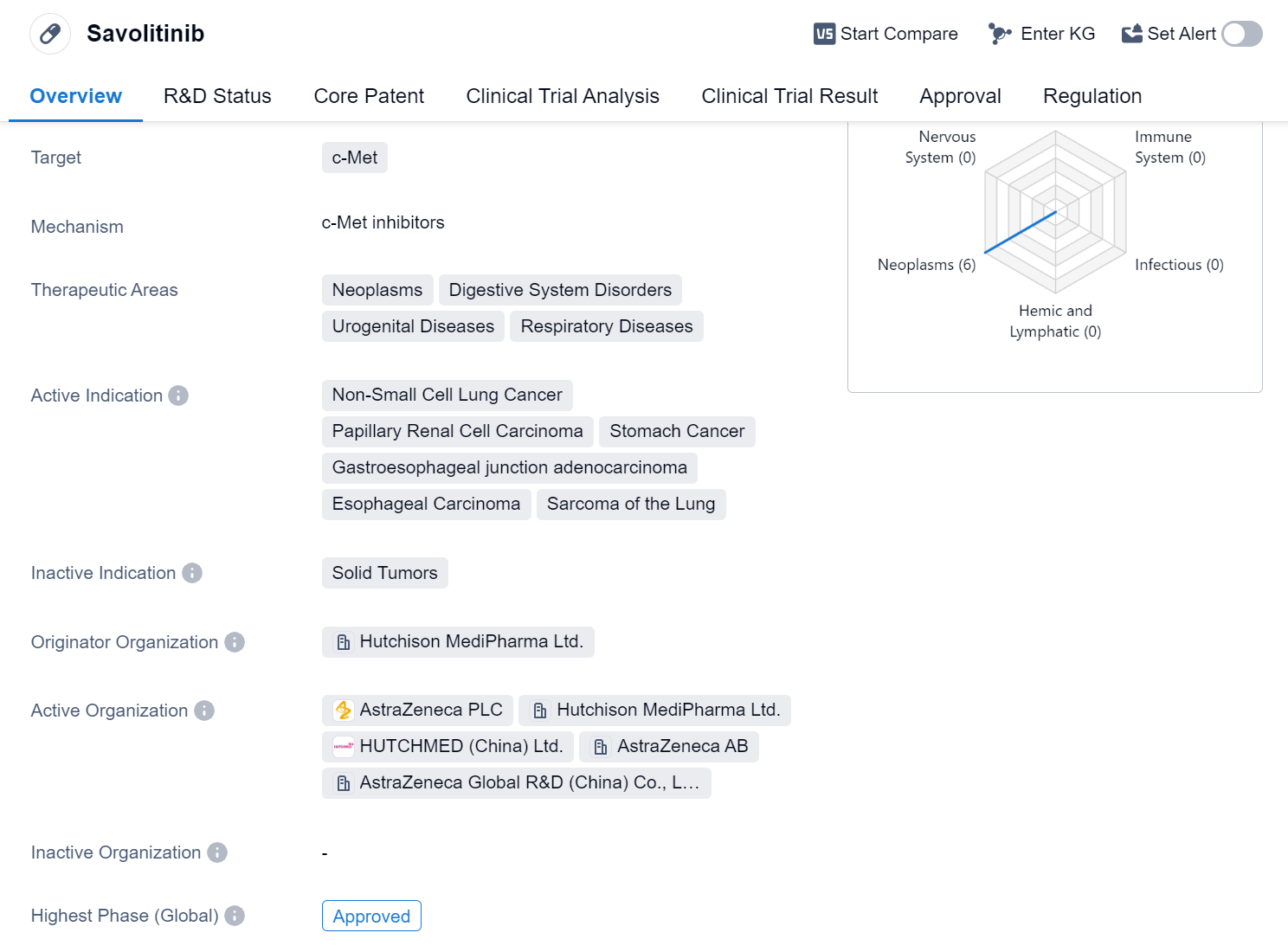
Savolitinib is a small molecule drug that falls under the category of biomedicine. It specifically targets c-Met, a protein that plays a crucial role in various diseases. The drug has shown potential therapeutic benefits in the treatment of neoplasms, digestive system disorders, urogenital diseases, and respiratory diseases.
In terms of active indications, Savolitinib has demonstrated efficacy in the treatment of several types of cancers. These include non-small cell lung cancer, papillary renal cell carcinoma, stomach cancer, gastroesophageal junction adenocarcinoma, esophageal carcinoma, and sarcoma of the lung. This wide range of indications highlights the drug's versatility and potential to address multiple cancer types.
Savolitinib was developed by Hutchison MediPharma Ltd., an originator organization specializing in pharmaceutical research and development. The drug has reached the highest phase of approval globally. It received its first approval in China in June 2021, marking a significant milestone for the drug and its potential availability to patients in that region.
Regulatory authorities have recognized the importance of Savolitinib and its potential to address unmet medical needs. The drug has been granted various regulatory designations, including priority review, conditional marketing approval, breakthrough therapy, and special review project. These designations indicate that Savolitinib has shown promising results in clinical trials and may offer significant benefits to patients in need.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Savolitinib: c-Met inhibitors
c-Met inhibitors are a type of medication that specifically target and inhibit the activity of the c-Met protein. The c-Met protein, also known as the hepatocyte growth factor receptor (HGFR), is a receptor tyrosine kinase that plays a crucial role in cell growth, survival, and migration. It is involved in various cellular processes, including tissue development, wound healing, and organ regeneration.
In a biomedical perspective, c-Met inhibitors are primarily used in the field of oncology. Abnormal activation of the c-Met pathway has been implicated in the development and progression of several types of cancer, including lung, liver, breast, and gastric cancers. By inhibiting the c-Met protein, these inhibitors can help to suppress the growth and spread of cancer cells.
c-Met inhibitors work by binding to the c-Met protein and preventing its activation, thereby interfering with the downstream signaling pathways that promote cell proliferation and survival. This inhibition can lead to the suppression of tumor growth, inhibition of angiogenesis (formation of new blood vessels to supply the tumor), and induction of apoptosis (programmed cell death) in cancer cells.
Some examples of c-Met inhibitors include crizotinib, cabozantinib, and capmatinib. These drugs have shown promising results in clinical trials and have been approved for the treatment of certain types of cancer. However, it is important to note that like any medication, c-Met inhibitors may have side effects and should be used under the guidance of a healthcare professional.
Drug Target R&D Trends for Savolitinib
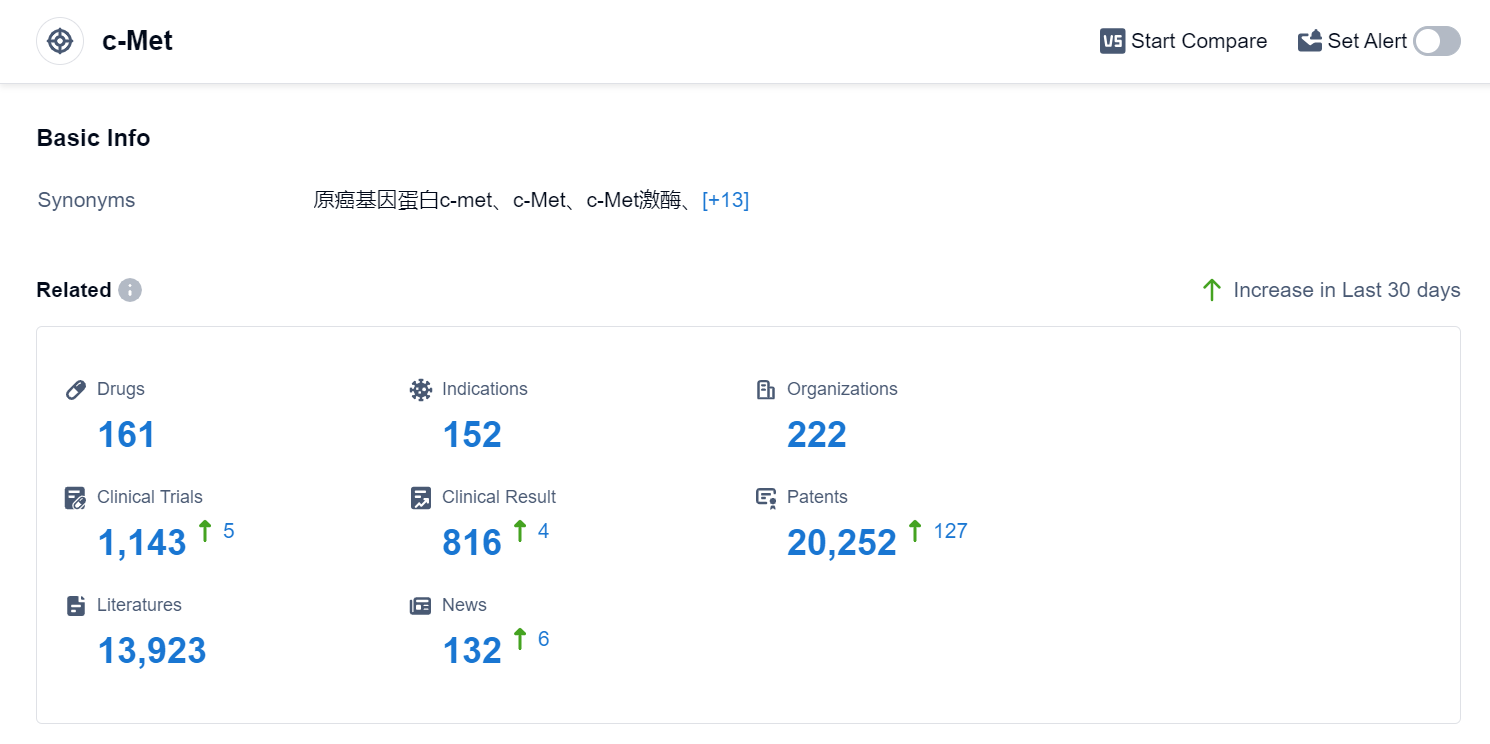
According to Patsnap Synapse, as of 4 Sep 2023, there are a total of 161 c-Met drugs worldwide, from 222 organizations, covering 152 indications, and conducting 1143 clinical trials.
The analysis of the current competitive landscape of target c-Met reveals that Pfizer Inc. and Johnson & Johnson are the leading companies in terms of R&D progress, with drugs in all phases of development. The indications for drugs targeting c-Met are primarily focused on various types of cancer, including Non-Small Cell Lung Cancer, Liver Cancer, Hepatocellular Carcinoma, Renal Cell Carcinoma, and Thyroid Cancer. Small molecule drugs, bispecific antibodies, and monoclonal antibodies are the most rapidly progressing drug types. China is showing significant progress in the development of drugs targeting c-Met. Overall, the target c-Met presents a competitive landscape with multiple companies and drug types, indicating a promising future for the development of innovative therapies in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Savolitinib is a small molecule drug developed by Hutchison MediPharma Ltd. It targets c-Met and has shown efficacy in the treatment of various cancers, digestive system disorders, urogenital diseases, and respiratory diseases. The drug has received approvals globally, with its first approval granted in China in June 2021. Regulatory authorities have recognized the potential of Savolitinib, granting it priority review, conditional marketing approval, breakthrough therapy, and special review project designations. These developments position Savolitinib as a promising option for patients in need of effective treatments for the indicated conditions.