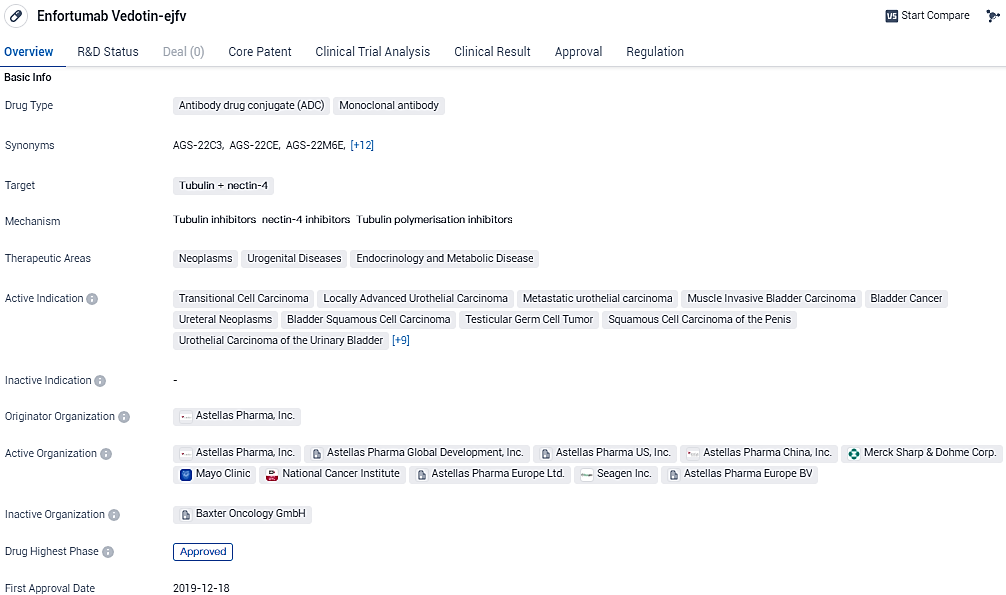
EMA Reviews Application for PADCEV® Plus KEYTRUDA® Combo as Initial Therapy for Progressive Bladder Cancer
On January 26, Pfizer Inc. together with Astellas Pharma Inc. reported that the European Medicines Agency has officially accepted to assess a Type II variation application concerning the joint use of PADCEV® (enfortumab vedotin) in combination with KEYTRUDA® (pembrolizumab). This application is for the proposed use as an initial therapy for adult individuals who have not yet been treated for locally advanced or metastatic urothelial carcinoma.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Upon authorization, the combination of PADCEV and KEYTRUDA could revolutionize current treatment approaches by providing a first-of-its-kind option that doesn't rely on chemotherapies based on platinum, which are traditionally used as the initial standard therapy for la/mUC.
Worldwide, each year sees the emergence of roughly 573,000 new cases of bladder cancer and a consequent 212,000 fatalities. Europe alone witnesses the diagnosis of bladder cancer in around 200,000 individuals annually.
"For individuals in Europe dealing with advanced bladder cancer, the survival rates are notably low, necessitating the introduction of groundbreaking treatments that prolong life. This milestone moves us one step closer towards fulfilling our goal: to provide transformative solutions that tackle the pressing needs of patients and revolutionize the current state of care for advanced urothelial cancer," stated Roger Dansey, M.D., Senior Vice President and the Head of Oncology Development at Pfizer.
The EMA’s Human Medicines Committee and following that, the European Commission, are anticipated to issue their evaluative feedback and final rulings pertaining to the submitted Type II variation application within the 2024 calendar year. Previously, in December 2023, the U.S. Food and Drug Administration granted approval for the use of this combination therapy.
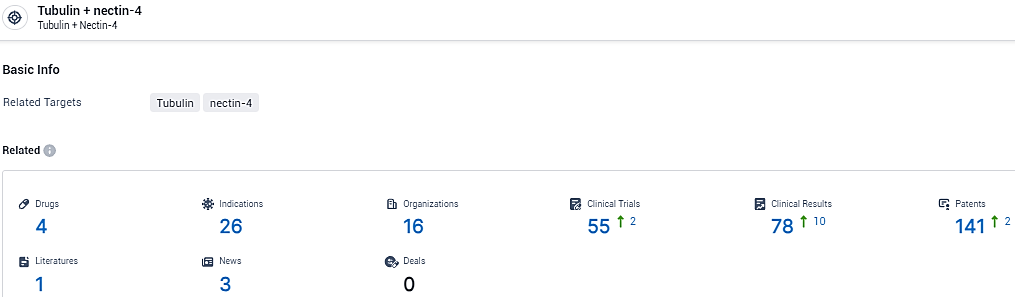
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 31, 2024, there are 4 investigational drugs for the Tubulin and nectin-4 target, including 26 indications, 16 R&D institutions involved, with related clinical trials reaching 55, and as many as 141 patents.
PADCEV (enfortumab vedotin) is a first-in-class antibody-drug conjugate that is directed against Nectin-4, a protein located on the surface of cells and highly expressed in bladder cancer. Enfortumab Vedotin represents a significant advancement in the field of biomedicine, offering a promising treatment option for various neoplasms and urogenital diseases. Its approval in the United States and ongoing regulatory processes in China indicate its potential to make a positive impact on patients.