Enterprise Therapeutics administers ETD001 to first cystic fibrosis patient in Phase 2 study
Enterprise Therapeutics Ltd, a biopharmaceutical company focused on finding and developing new treatments to enhance the lives of individuals with respiratory illnesses, has today revealed that the first participant with cystic fibrosis has been administered a dose in its Phase 2a study of ETD001.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
 ETD001, a pioneering low molecular weight compound, aims at the epithelial sodium channel in the airway epithelium to enhance mucus hydration and clearance. The Phase 2a trial is designed to establish clinical proof-of-concept and evaluate the safety profile of ETD001 in the 10% of people with cystic fibrosis (pwCF) who have the most critical unmet medical needs. This study will be conducted at various locations in the UK, Germany, France, and Italy, focusing on lung function in pwCF who are either unsuitable for or not currently receiving CFTR modulators.
ETD001, a pioneering low molecular weight compound, aims at the epithelial sodium channel in the airway epithelium to enhance mucus hydration and clearance. The Phase 2a trial is designed to establish clinical proof-of-concept and evaluate the safety profile of ETD001 in the 10% of people with cystic fibrosis (pwCF) who have the most critical unmet medical needs. This study will be conducted at various locations in the UK, Germany, France, and Italy, focusing on lung function in pwCF who are either unsuitable for or not currently receiving CFTR modulators.
Cystic fibrosis (CF) is estimated to impact over 100,000 individuals globally, with an average life expectancy of just 50 years. Impaired mucociliary clearance and mucus buildup in the lungs of pwCF result in recurrent infections and inflammation, contributing to progressive lung function decline. Enhancing fluid volume in the lungs by inhibiting ENaC with ETD001 will hydrate mucus, improve its clearance, reduce congestion, and is anticipated to significantly enhance lung function. In earlier Phase 1 trials, ETD001 showed a robust safety profile in healthy participants and demonstrated long-acting properties in pre-clinical studies.
Dr. John Ford, CEO of Enterprise Therapeutics, stated, “Administering the first dose of ETD001 to a CF patient in our Phase 2a trial is a remarkable milestone, reflecting Enterprise’s commitment to developing a novel treatment approach for pwCF with the greatest unmet medical needs. ETD001 has already proven itself with an excellent safety profile in healthy participants and a pharmacokinetic (PK) profile indicating prolonged lung residency. We are eager to advance ETD001 through Phase 2 trials and beyond.”
Paul Russell, Head of Development at Enterprise Therapeutics, noted, “By addressing the core mechanisms of mucus congestion in the lungs via ENaC inhibition, ETD001 holds potential as an innovative respiratory therapeutic. This not only applies to the ~10% of pwCF who are not suitable for or do not benefit from CFTR modulators but also extends to individuals suffering from other muco-obstructive lung conditions such as COPD and asthma. Initiating our Phase 2a trial is a thrilling step towards achieving this potential.”
Dr. Renu Gupta, CMO of Enterprise Therapeutics, commented, “We are thankful to the pwCF participating in our Phase 2 study of ETD001 and to the clinical investigators for reaching this significant milestone. We are optimistic that our unwavering dedication to developing this cutting-edge ENaC-targeting molecule, along with our collaborations with the CF community, will culminate in a treatment that will drastically improve the lives of those living with CF.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
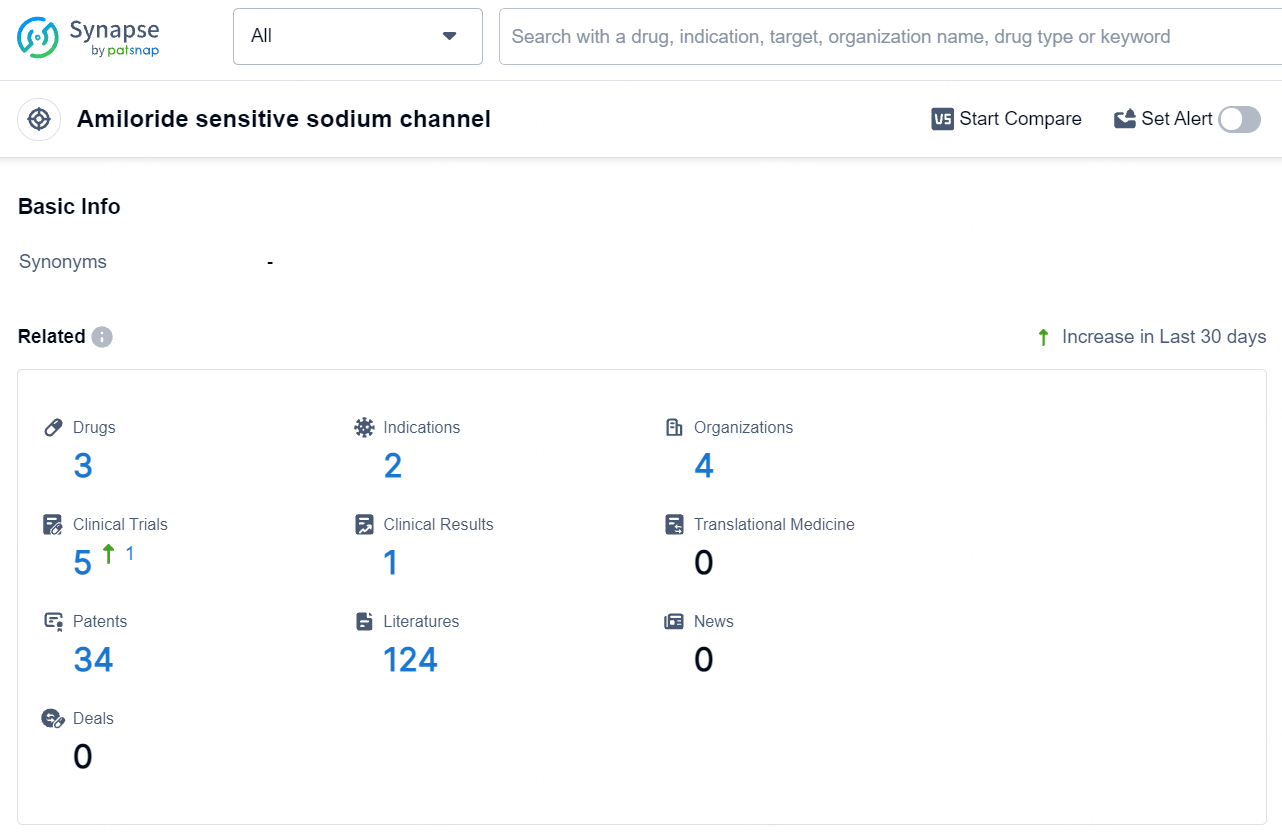
According to the data provided by the Synapse Database, As of July 25, 2024, there are 3 investigational drugs for the amiloride sensitive sodium channel target, including 2 indications, 4 R&D institutions involved, with related clinical trials reaching 5, and as many as 34 patents.
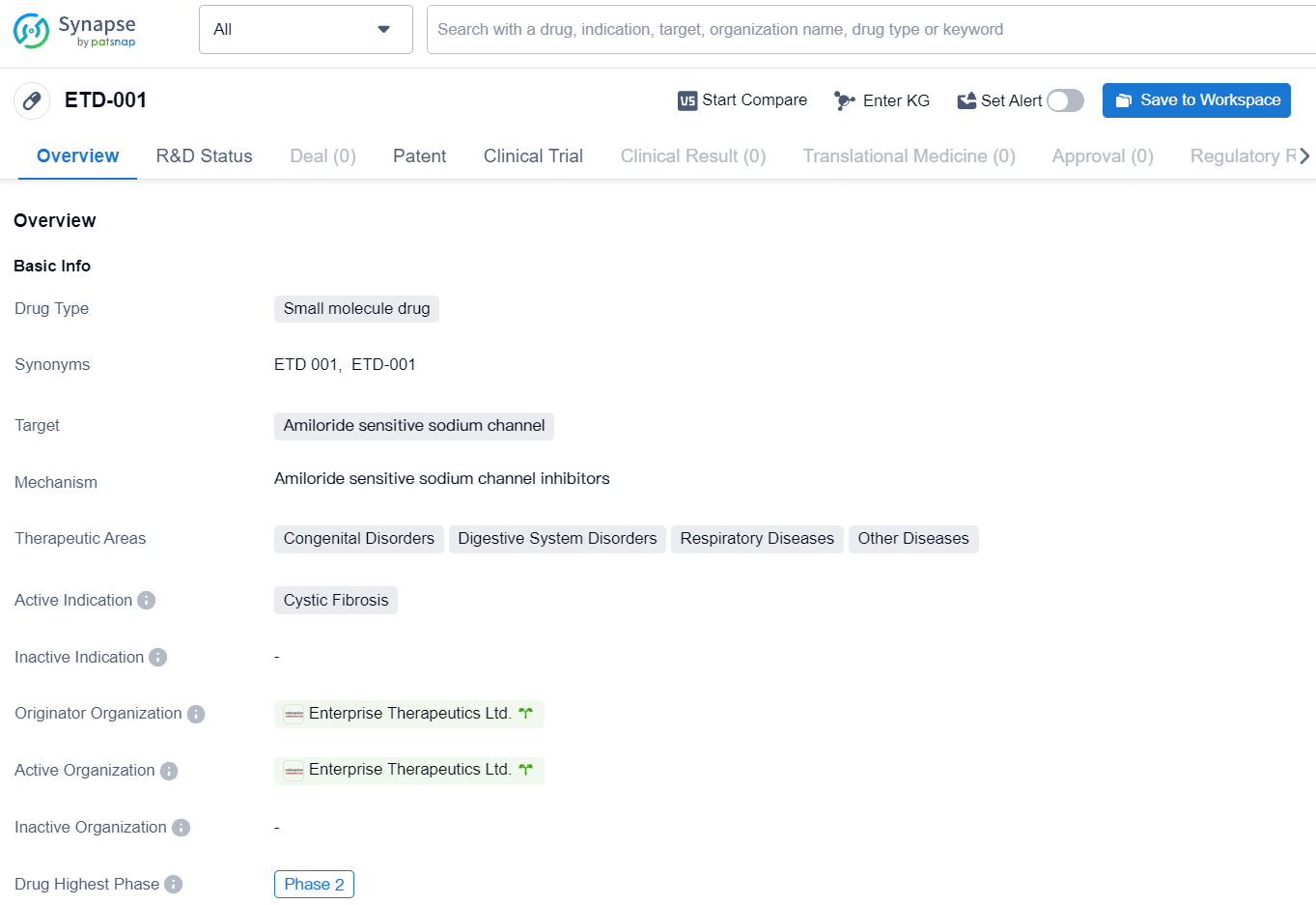
ETD-001 is a small molecule drug that targets the amiloride sensitive sodium channel. The drug is being developed for the treatment of cystic fibrosis, a genetic disorder that affects the respiratory and digestive systems. ETD-001 represents a novel approach in the treatment of cystic fibrosis and has the potential to address unmet medical needs in the field of congenital disorders, digestive system disorders, and respiratory diseases. As the drug progresses through clinical development, ongoing research and development efforts will continue to shed light on its therapeutic benefits and its potential to improve patient outcomes in these challenging disease areas.