Epirium Bio's MF-300 Gets FDA Approval; Alex Casdin Named CEO, Russell Cox as Executive Chairman
Epirium Bio, Inc. (Epirium), a biopharmaceutical firm focused on developing treatments for neuromuscular and fibrotic disorders, is excited to share two important milestones that signify a new phase for the Company. The U.S. Food and Drug Administration (FDA) has approved the Company's Investigational New Drug (IND) application for MF-300, which is the first oral inhibitor targeting the 15-hydroxyprostaglandin dehydrogenase (15-PGDH) enzyme, currently under investigation for treating sarcopenia, defined as muscle weakness related to aging. The initial study launched under this IND will be a Phase 1 clinical trial, which is randomized, double-blinded, placebo-controlled, and focused on dose escalation involving healthy participants. The main goal of the Phase 1 trial is to assess the safety and tolerability of orally administered MF-300 among healthy individuals. Additional aims of the trial include analyzing the pharmacokinetics of MF-300 and establishing a recommended dose for Phase 2 studies. Recruitment of patients for the dose escalation segment of this Phase 1 study is set to begin in December 2024.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Epirium has announced simultaneous leadership changes, including the transition of Russell Cox from President and CEO to Executive Chairman, effective January 1, 2025. Alex Casdin, currently the Chief Operating Officer of Epirium, will assume the role of CEO on the same date. “Transitioning to a clinical stage company is a pivotal moment for Epirium, and I am happy to hand the CEO position to Alex, who has been vital in advancing the Company’s development thus far,” stated Russell Cox, the outgoing CEO.
With 25 years of experience in creating value across various firms in the biotech, biopharmaceutical, and healthcare industries, Mr. Casdin possesses a deep reservoir of knowledge and skills for his new position. “I am excited to accept the role of CEO during such a thrilling period for our company. I anticipate leading Epirium as we advance into clinical trials. I also want to thank our committed and talented team for their persistent efforts and strong focus on delivering first-in-class oral treatments aimed at neuromuscular disorders with significant unmet medical needs like sarcopenia,” remarked Mr. Casdin. “I look forward to continuing collaboration with Russ and our Board to generate substantial value for Epirium’s stakeholders as we enter this new phase.”
Building on its operational success, Epirium obtained a bridge financing round earlier this month, supported by its existing investor group. “This influx of capital will allow Epirium to report MF-300 Phase 1 results in the second half of 2025 and to make essential investments to facilitate the timely start of a Phase 2 study in sarcopenia patients by the first half of 2026,” remarked Paul Berns, Chairman of the Board of Directors.
“We are very optimistic about the preclinical findings showing enhanced muscle force and eagerly await results from human trials. I also want to express my appreciation to Russ for his unwavering dedication and commitment to Epirium’s goal of advancing MF-300 into the clinical stage,” added Patrick Enright, Co-Lead Investor at Longitude Capital.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
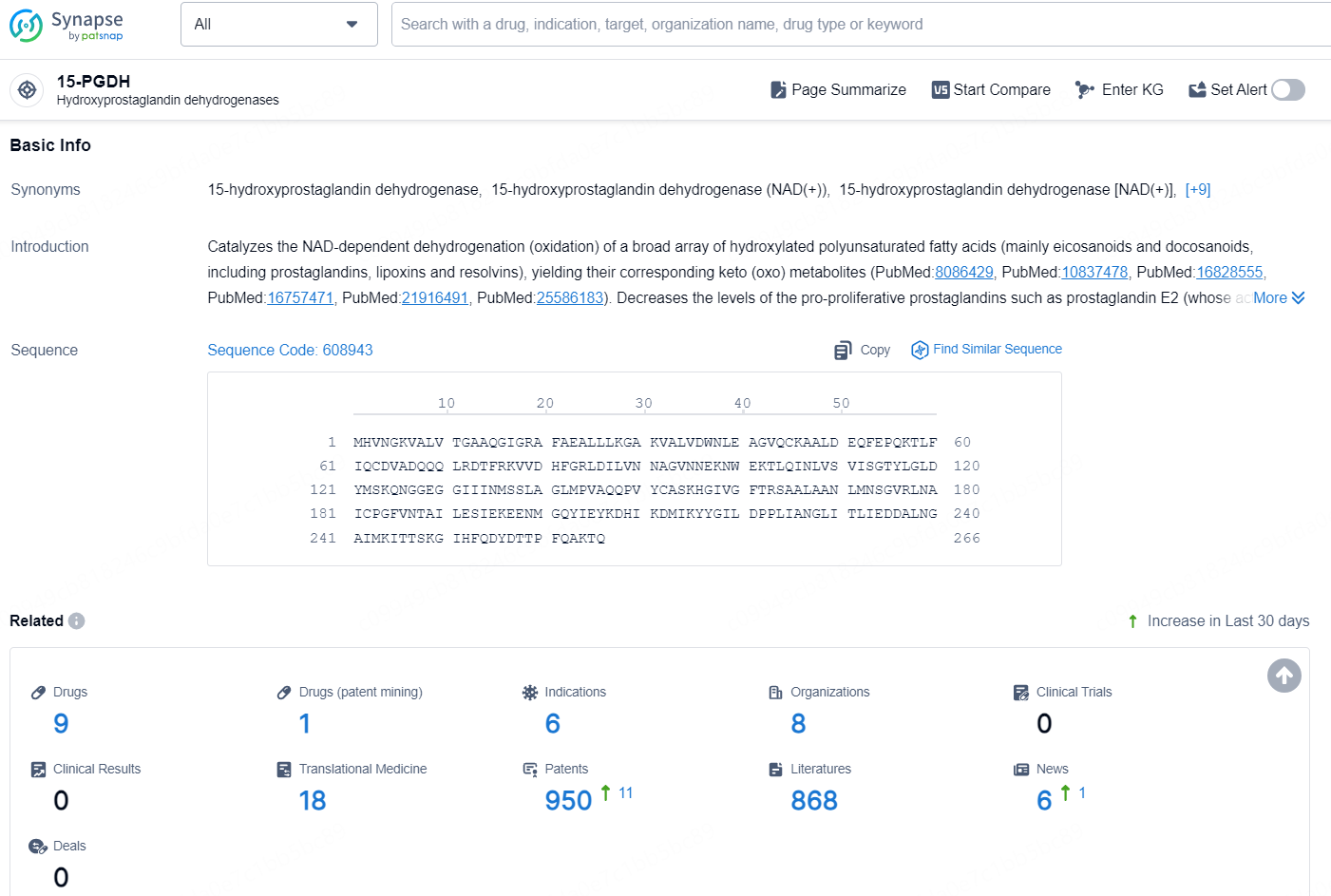
According to the data provided by the Synapse Chemical, As of December 30, 2024, there are 9 investigational drugs for the 15-PGDH target, including 6 indications, 8 R&D institutions involved, and as many as 950 patents.
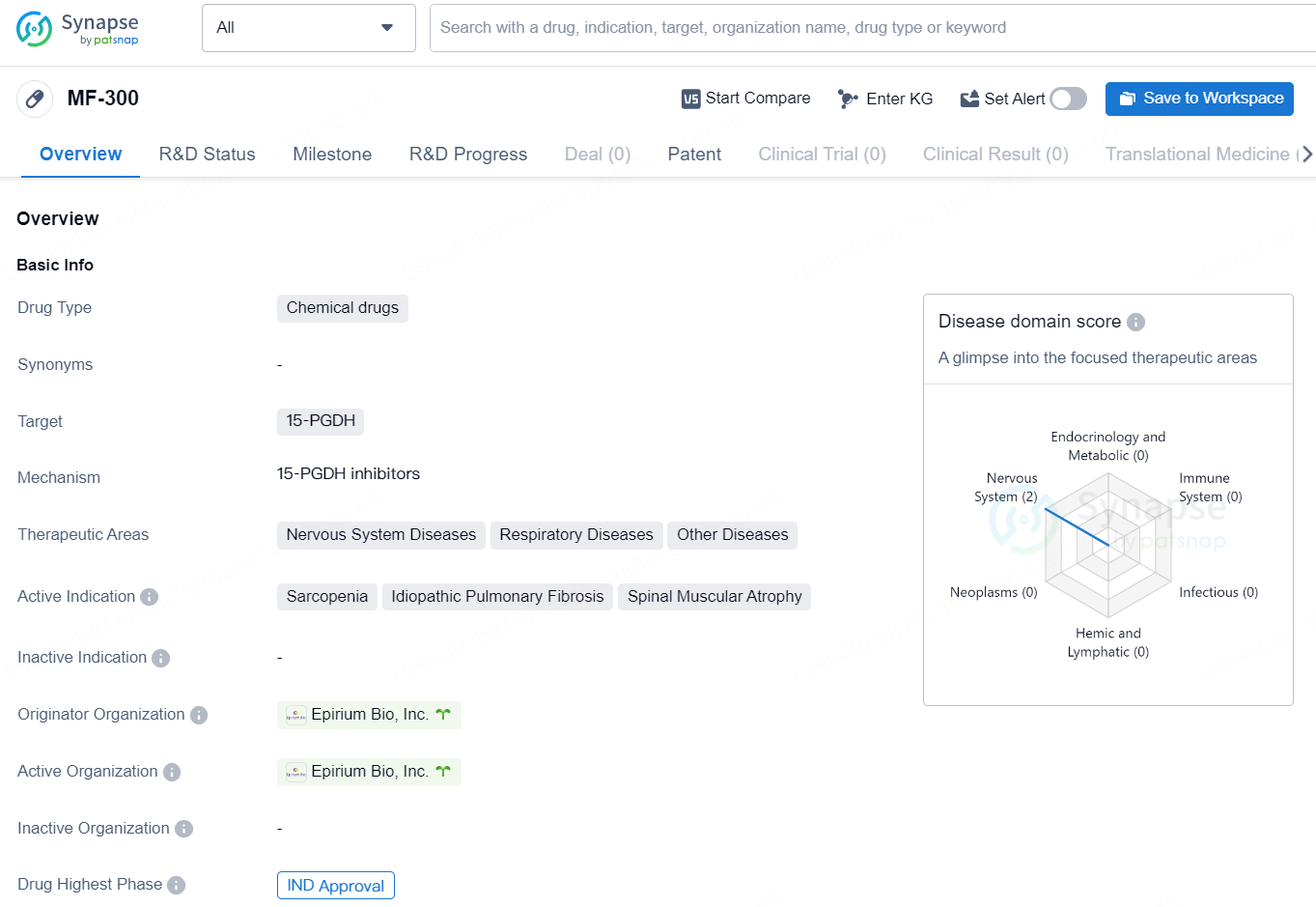
The drug MF-300 is classified as a chemical drug and targets the enzyme 15-PGDH. It is designed to treat a range of therapeutic areas including Nervous System Diseases, Respiratory Diseases, and Other Diseases. The active indications for MF-300 include Sarcopenia, Idiopathic Pulmonary Fibrosis, and Spinal Muscular Atrophy. The drug is developed by Epirium Bio, Inc., the originator organization, and has received IND Approval, representing the highest phase of development at a global level.