EU Approval Granted for Ordspono™ (odronextamab) to Treat Two Types of Lymphoma
Regeneron Pharmaceuticals, Inc. (NASDAQ: REGN) revealed that the European Commission (EC) has authorized the use of Ordspono™ (odronextamab) for the treatment of adult patients with relapsed or refractory (R/R) follicular lymphoma (FL) or R/R diffuse large B-cell lymphoma (DLBCL) after they have had at least two lines of systemic therapy. This is the inaugural global regulatory approval for Ordspono for these conditions. Ordspono is a bispecific antibody that functions by connecting the lymphoma cell to a cytotoxic T cell.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Dr. Stefano Luminari, M.D., an Oncology Professor at the University of Modena and Reggio Emilia, and hematologist at Arcispedale Sant Maria Nuova in Reggio Emilia, announced, “The EC approval of Ordspono represents a significant step forward for patients and physicians in the EU, providing a new treatment option for both indolent and aggressive lymphomas. Ordspono has shown impressive complete response rates in follicular lymphoma in clinical trials, and strong efficacy in diffuse large B-cell lymphoma, including post-CAR-T settings. This drug offers an outpatient, off-the-shelf option potentially leading to complete remission, especially crucial in community healthcare settings.”
The approval is supported by results from the Phase 1 ELM-1 and pivotal Phase 2 ELM-2 trials, which showed strong and lasting response rates in adults with R/R FL or R/R DLBCL:
For R/R FL, findings from ELM-2 (N=128), as assessed by an independent review committee (IRC), displayed an objective response rate (ORR) of 80%, with 73% achieving a complete response (CR). The median duration of response (DoR) for complete responders was 25 months (95% confidence interval [CI]: 20 months to not estimable [NE]).
For R/R DLBCL:
In patients naive to CAR-T therapy, results from ELM-2 (N=127), evaluated by an IRC, showed a 52% ORR with 31% achieving CR. The median DoR among complete responders was 18 months (95% CI: 10 months to NE).
In patients who had progressed after CAR-T therapy, results from ELM-1 (N=60), evaluated by an IRC, demonstrated a 48% ORR with 32% achieving CR. Among responders (n=29), the median DoR was 15 months (95% CI: 3 months to NE).
Common adverse reactions included cytokine release syndrome (CRS; 54%), neutropenia (41%), pyrexia (39%), anemia (38%), thrombocytopenia (27%), diarrhea (24%), and COVID-19 (22%). Serious adverse reactions frequently encountered were CRS (14%), pneumonia (9%), COVID-19 (9%), and pyrexia (6%). Ordspono can result in serious or fatal infections, and CRS, which may be severe or life-threatening.
George D. Yancopoulos, M.D., Ph.D., Board co-Chair, President, and Chief Scientific Officer of Regeneron stated, “Ordspono is our first approval from the bispecific antibody platform, which we anticipate will progressively add to our portfolio of transformative medicines for oncology and other diseases. Following this approval, we’re enthusiastic about our OLYMPIA program, which encompasses multiple Phase 3 trials evaluating Ordspono as a standalone treatment, as well as in various combinations, in earlier therapy lines. We're also eager to advance our broader pipeline of CD3 and other bispecific therapies, targeting additional hematologic cancers, such as myeloma, and also solid tumors.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
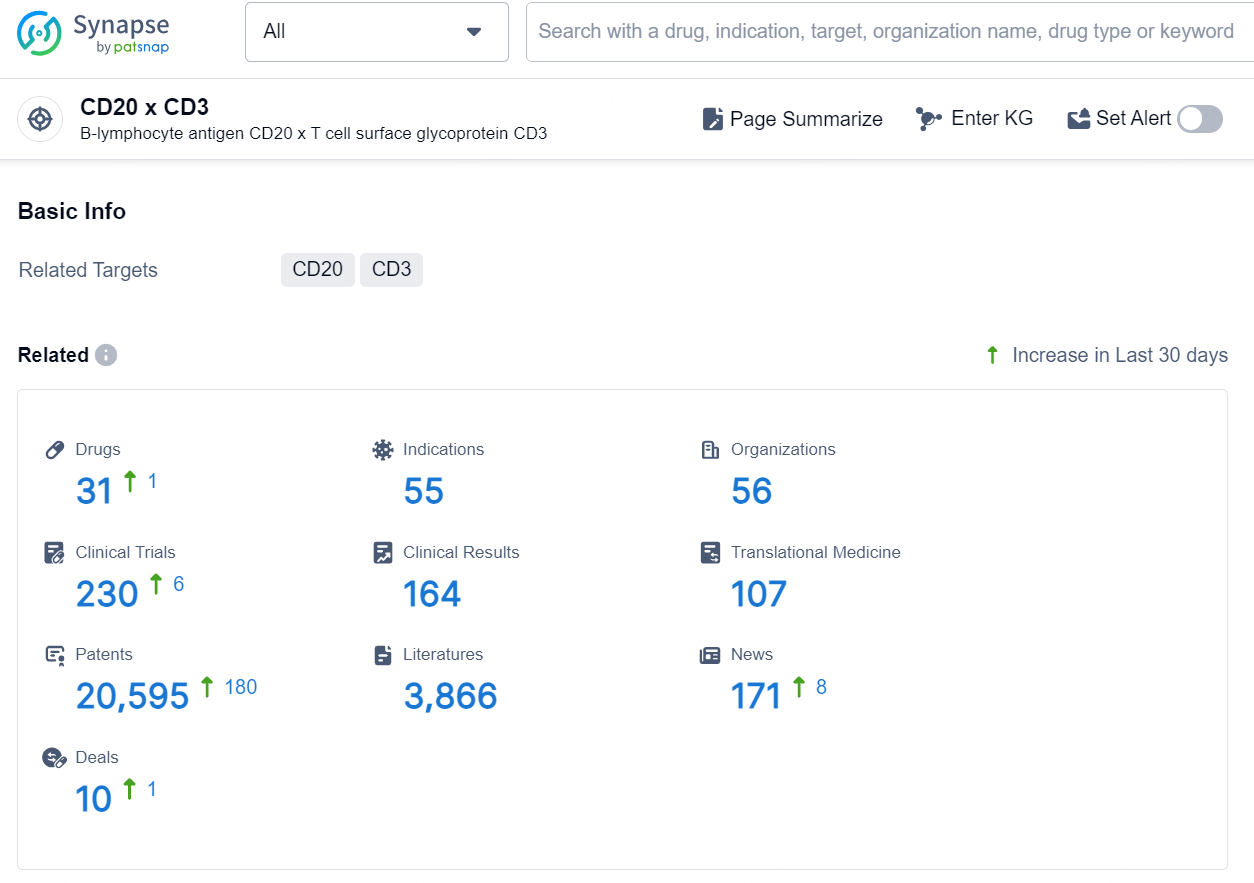
According to the data provided by the Synapse Database, As of August 29, 2024, there are 31 investigational drugs for the CD20 x CD3 targets, including 55 indications, 56 R&D institutions involved, with related clinical trials reaching 230, and as many as 20595 patents.
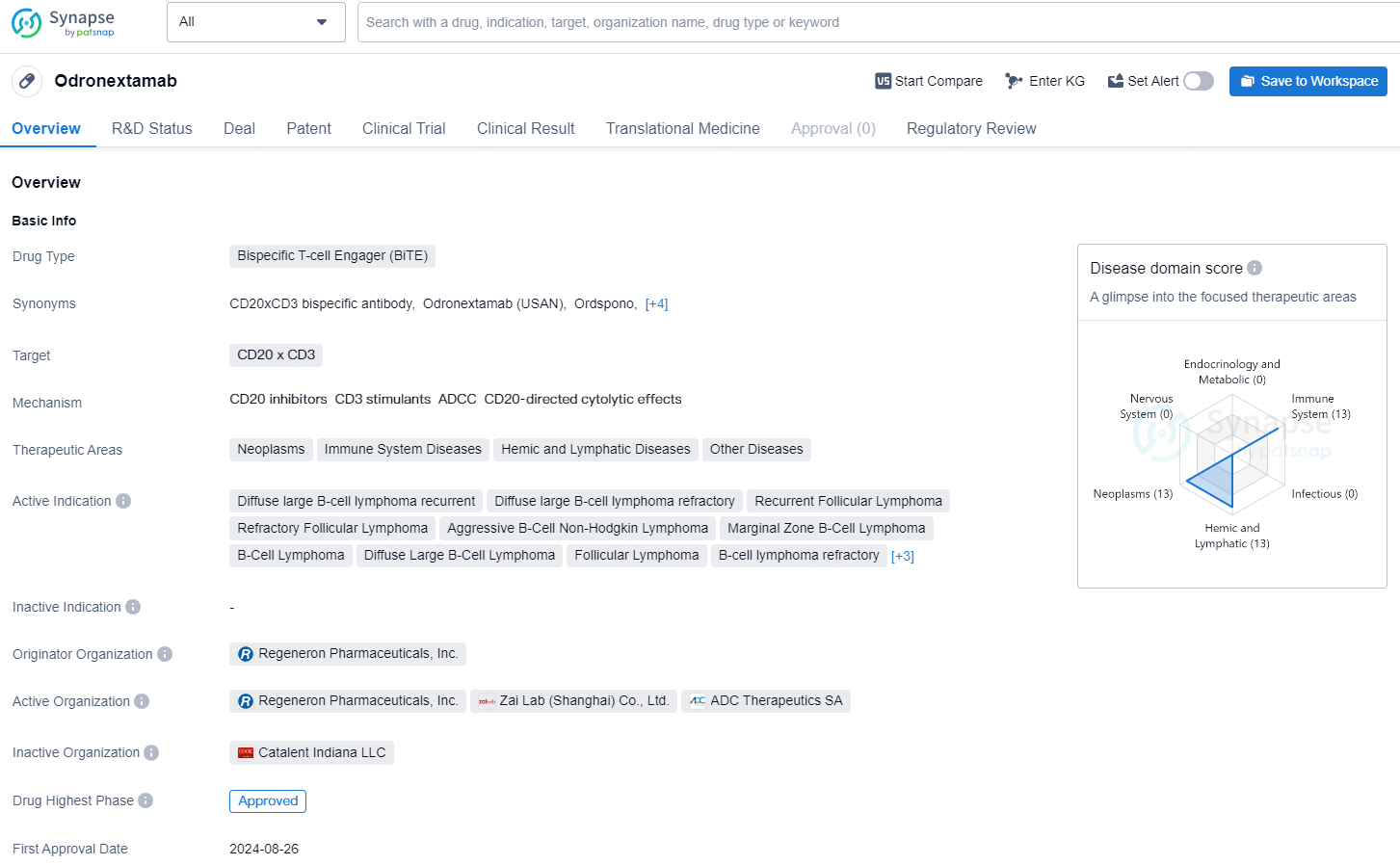
Odronextamab is a bispecific T-cell engager (BiTE) drug developed by Regeneron Pharmaceuticals, Inc. It targets the CD20 x CD3 receptors and is indicated for the treatment of various neoplasms, immune system diseases, hemic and lymphatic diseases, and other related conditions. The drug has been approved for use in the treatment of Diffuse large B-cell lymphoma recurrent, Diffuse large B-cell lymphoma refractory, Recurrent Follicular Lymphoma, Refractory Follicular Lymphoma, Aggressive B-Cell Non-Hodgkin Lymphoma, Marginal Zone B-Cell Lymphoma, B-Cell Lymphoma, Diffuse Large B-Cell Lymphoma, Follicular Lymphoma, B-cell lymphoma refractory, Non-Hodgkin Lymphoma, CD20 positive B-Cell Lymphoma, and Chronic Lymphocytic Leukemia.