siRNA Drug Amvuttra Patent Research and Practical Operation Guide(2)
Based on the characteristics of siRNA drugs, we have summarized the process for conducting patent research on siRNA drugs. You can start by reading the following article to get background information.
siRNA Drug Amvuttra Patent Research and Practical Operation Guide(1)
Amvuttra is a chemically modified, double-stranded small interfering RNA (siRNA) targeting mutant and wild-type transthyretin (TTR) messenger RNAs (mRNAs). It is covalently linked to a ligand containing three N-acetygalactosamine (GalNAc) residues, enabling the delivery of siRNAs to liver cells. Amvuttra causes the degradation of mutant and wild-type TTR mRNAs through RNA interference, thereby reducing the deposition of serum TTR protein and TTR protein in tissues.
As Alnylam's blockbuster product, Amvuttra generated $560 million in revenue in 2023. Compared with Alnylam's other TTR-targeting siRNA drug Onpattro, Amvuttra has lower manufacturing costs, eliminates the need of frequent administration, and has the convenience of subcutaneous injection Thus, it is expected that patients will further turn to Amvuttra.
This guide will demonstrate how to conduct patent research and analysis for Amvuttra, and includes the most recent data as of May 29, 2024.
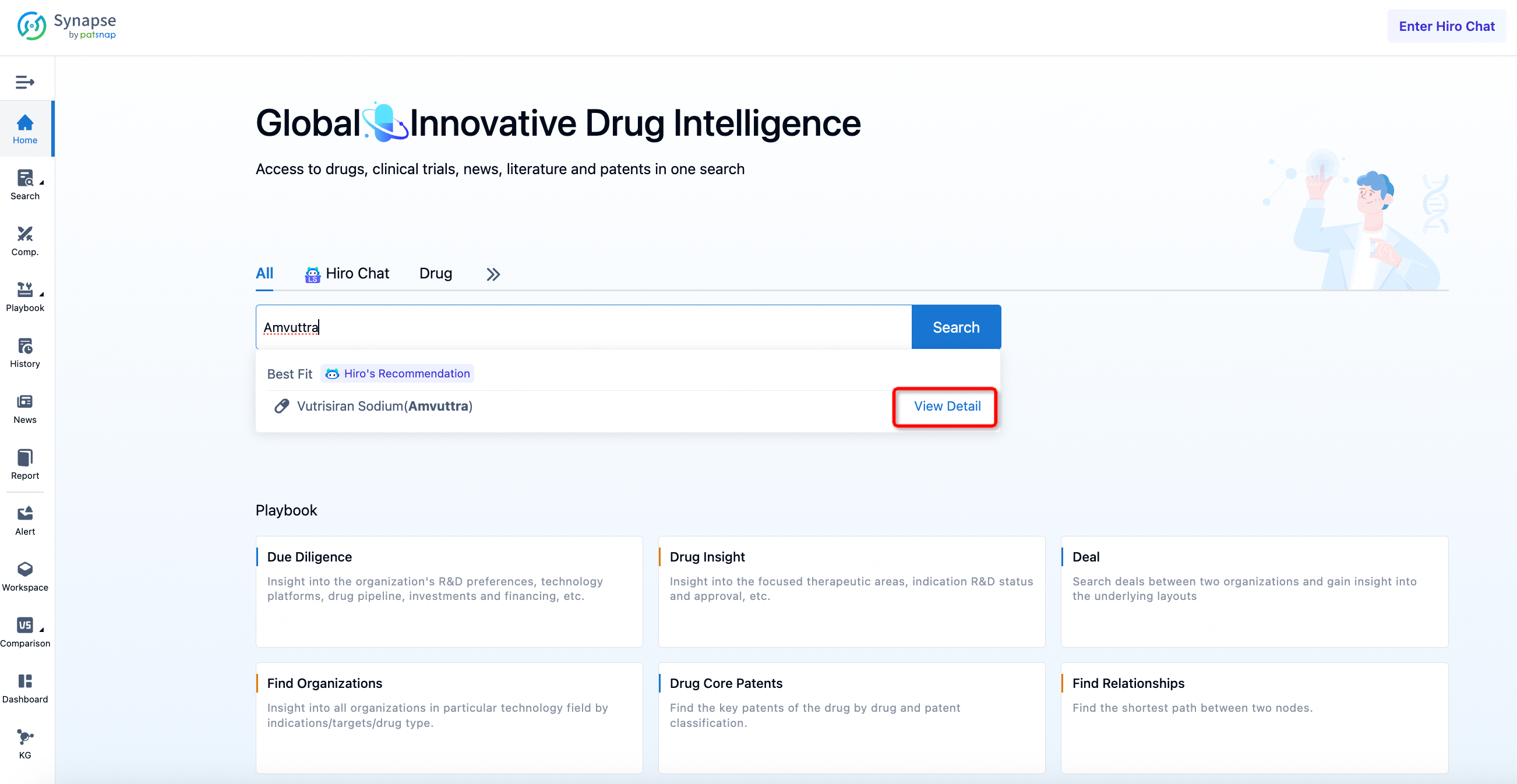
Retrieval of Basic Information on Amvuttra
First, log in to Patsnap Synapse, enter the drug "Amvuttra" in the homepage search bar, and click "Search". Next, click on the retrieved vutrisiran (Amvuttra) drug card and navigate to the "Overview" section to gather basic information regarding Amvuttra.
The compilation of basic information regarding Amvuttra
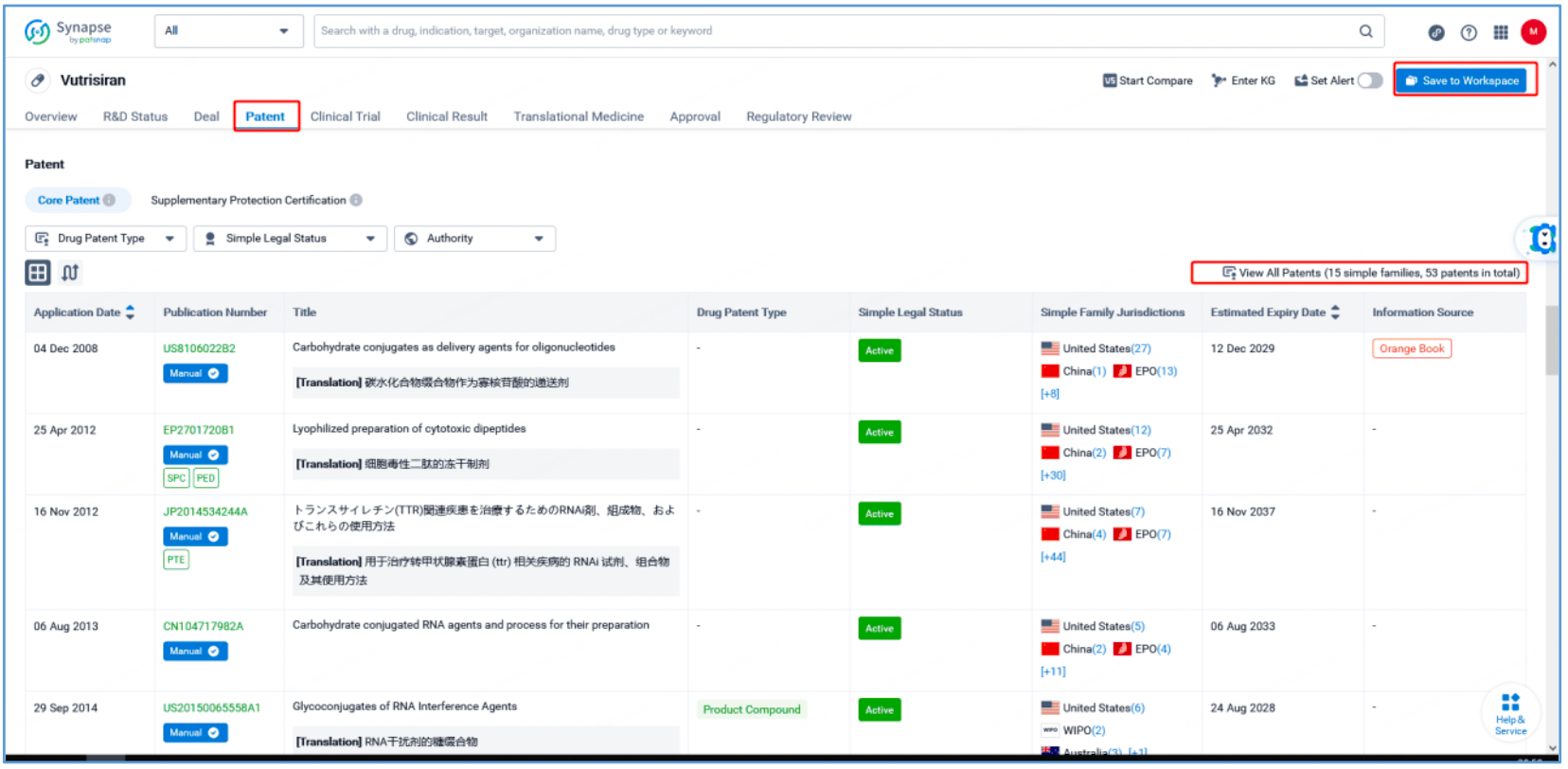
Preliminary Retrieval of Amvuttra-Related Patents and Core Patents Through Patsnap Synapse
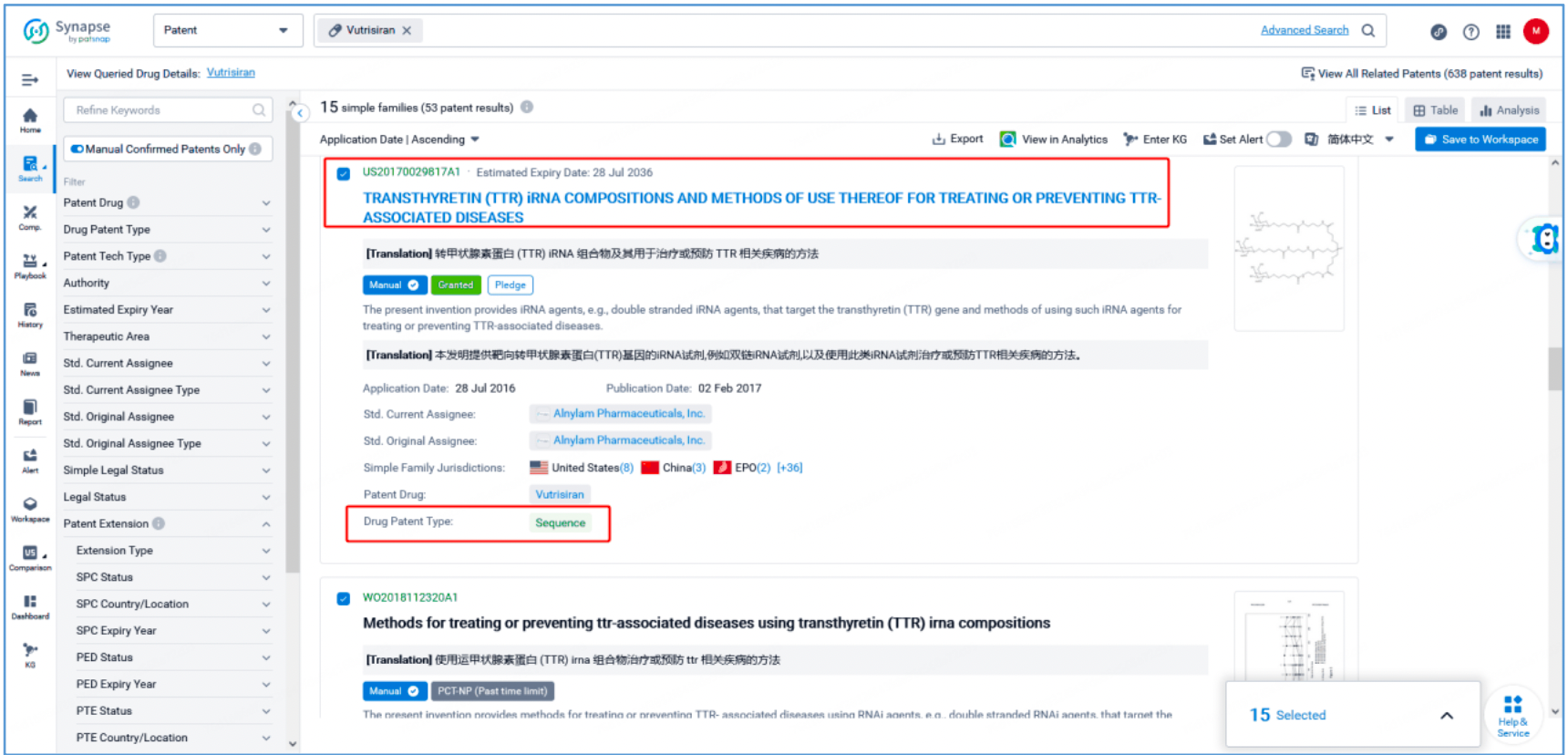
From the vutrisiran page, select the "Patent" tab and "View All Patents" on the right to retrieve all patents associated with vutrisiran. Select the 15 resulting simple families (with 53 patent results) and save them to a new Workspace. To monitor new changes to this group of patents, select ‘Set Alert’to create a custom email alert.
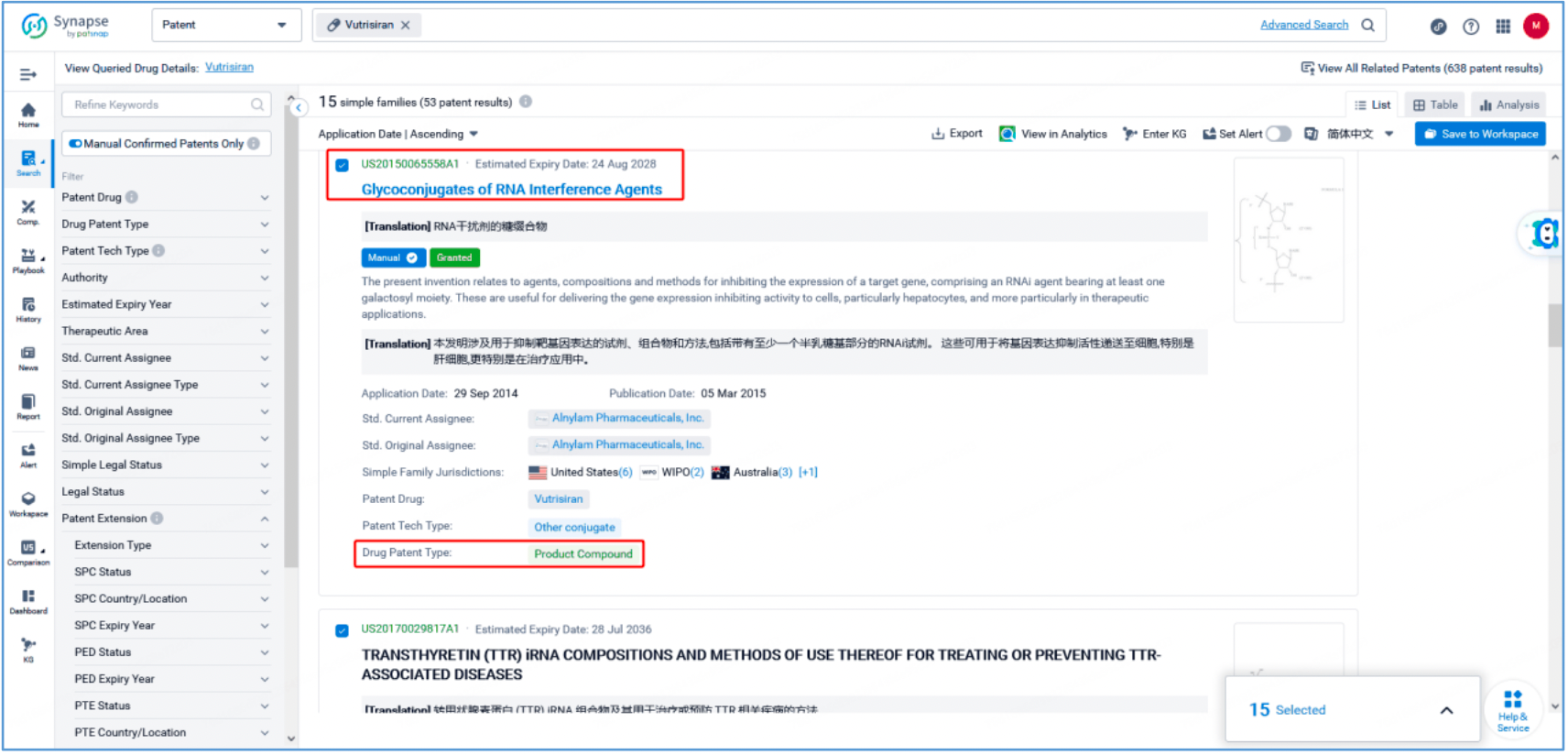
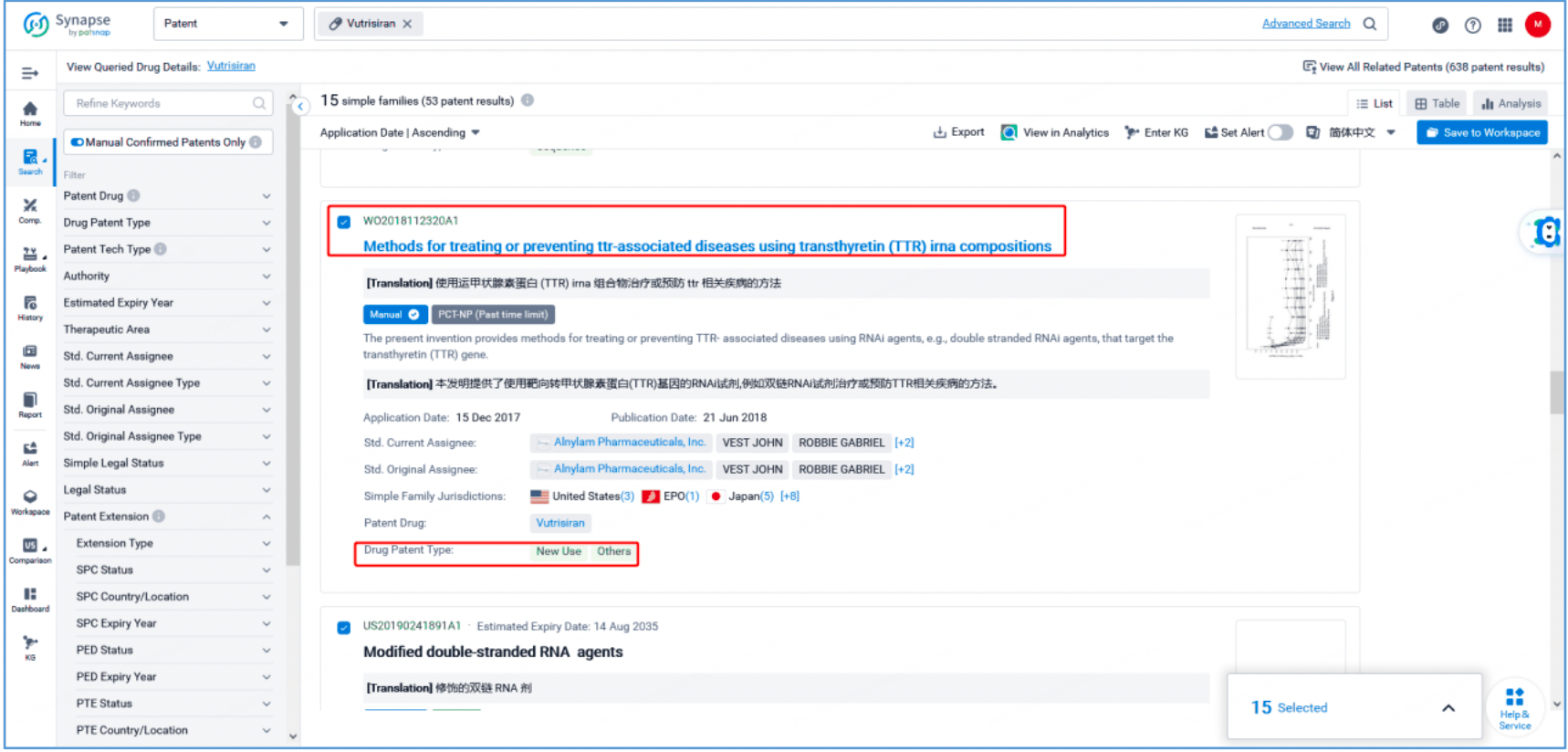
As seen from Synapse’s manual indexing, the following three patent families can be preliminarily identified as core product patent families: invention patent application US20150065558A1, "Glycoconjugates of RNA Interference Agents"; invention patent application US20170029817A1, "TRANSTHYRETIN (TTR) iRNA COMPOSITIONS AND METHODS OF USE THEREOF FOR TREATING OR PREVENTING TTR-ASSOCIATED DISEASES"; and PCT invention patent application WO2018112320A1, "Methods for treating or preventing ttr-associated diseases using transthyretin (TTR) irna compositions". The above patent families are classified as Product Compound, Sequence, and New Use patent types, respectively.
Analysis of Amvuttra Core Product Patents to Ascertain Composition of Each Component
Next, launch Patsnap Analytics to perform an in-depth analysis of the claims in each member of the three selected core product patent families. This will help identify the main protected sequences and confirm the composition of each component of Amvuttra.
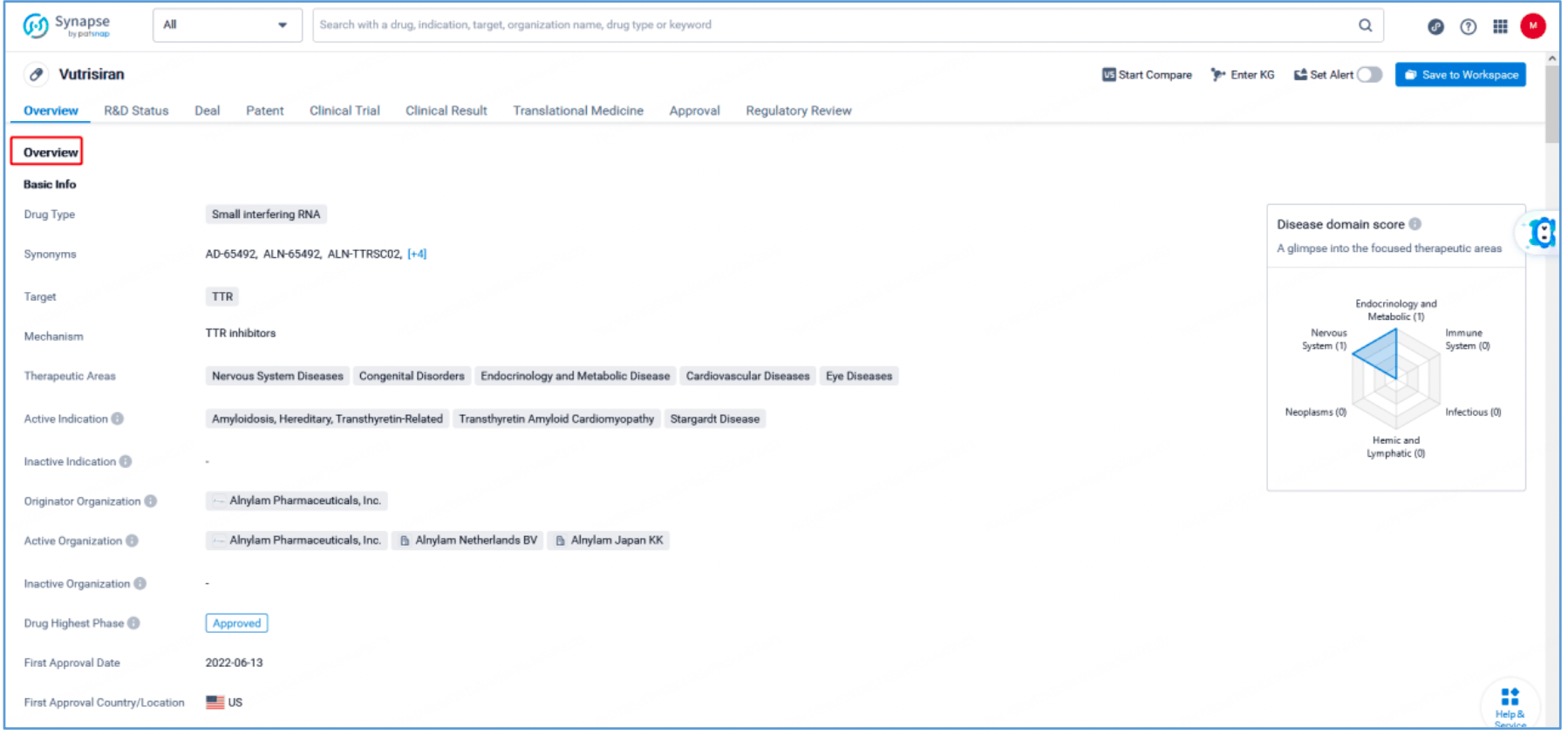
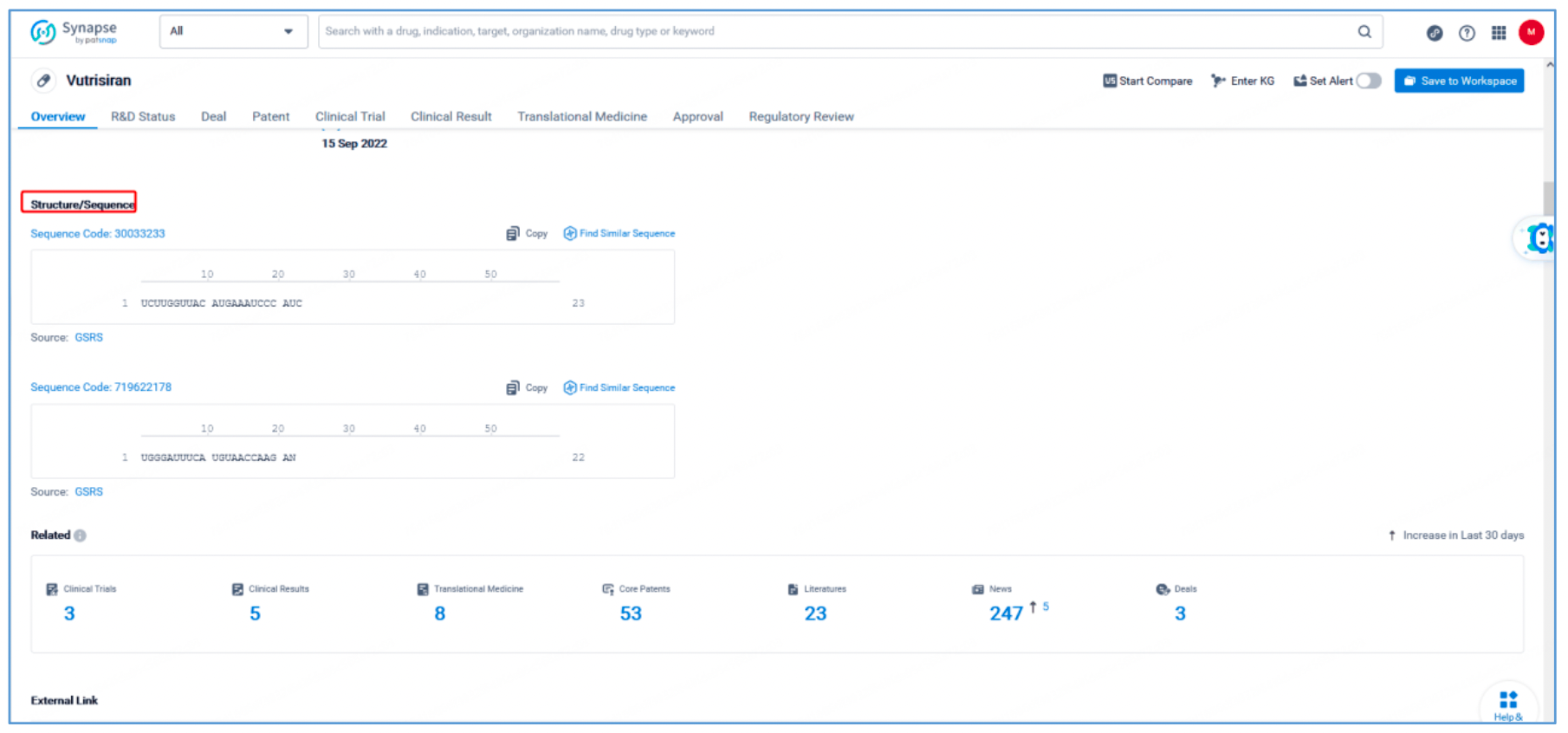
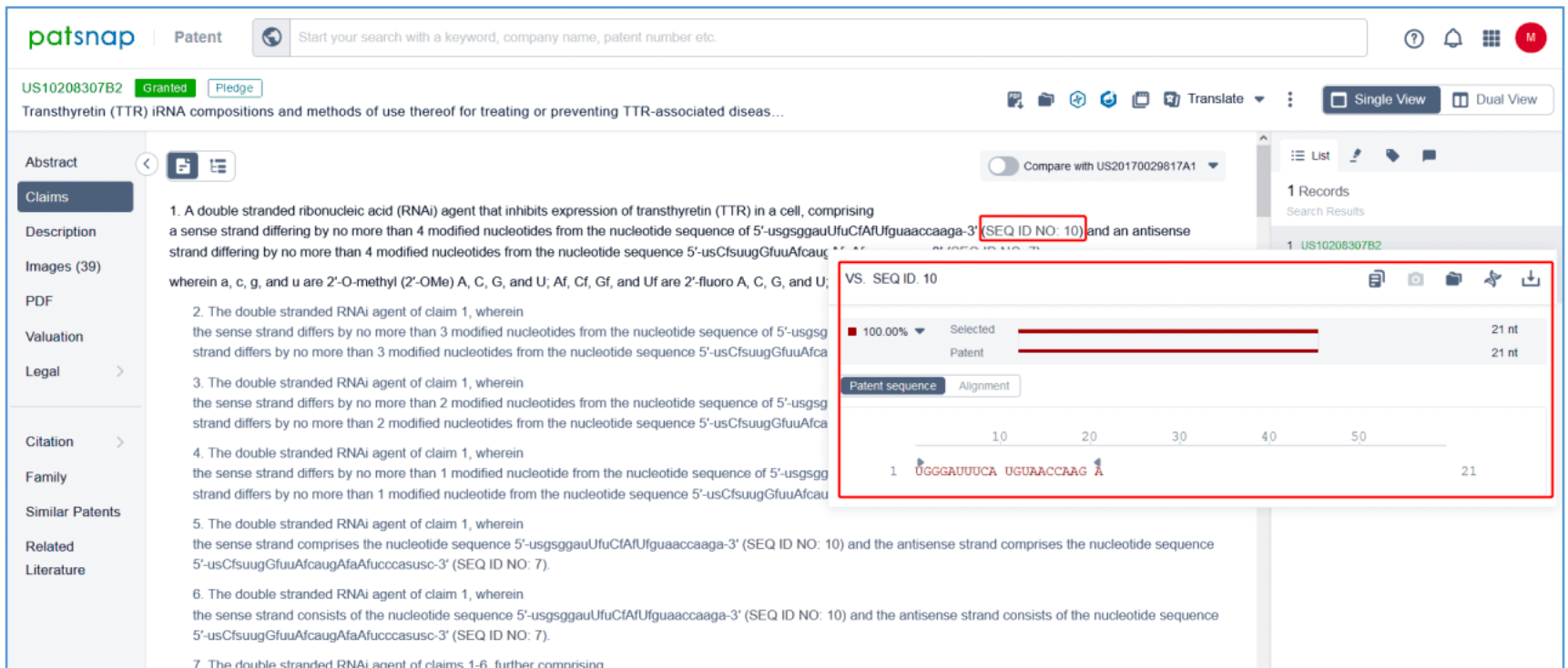
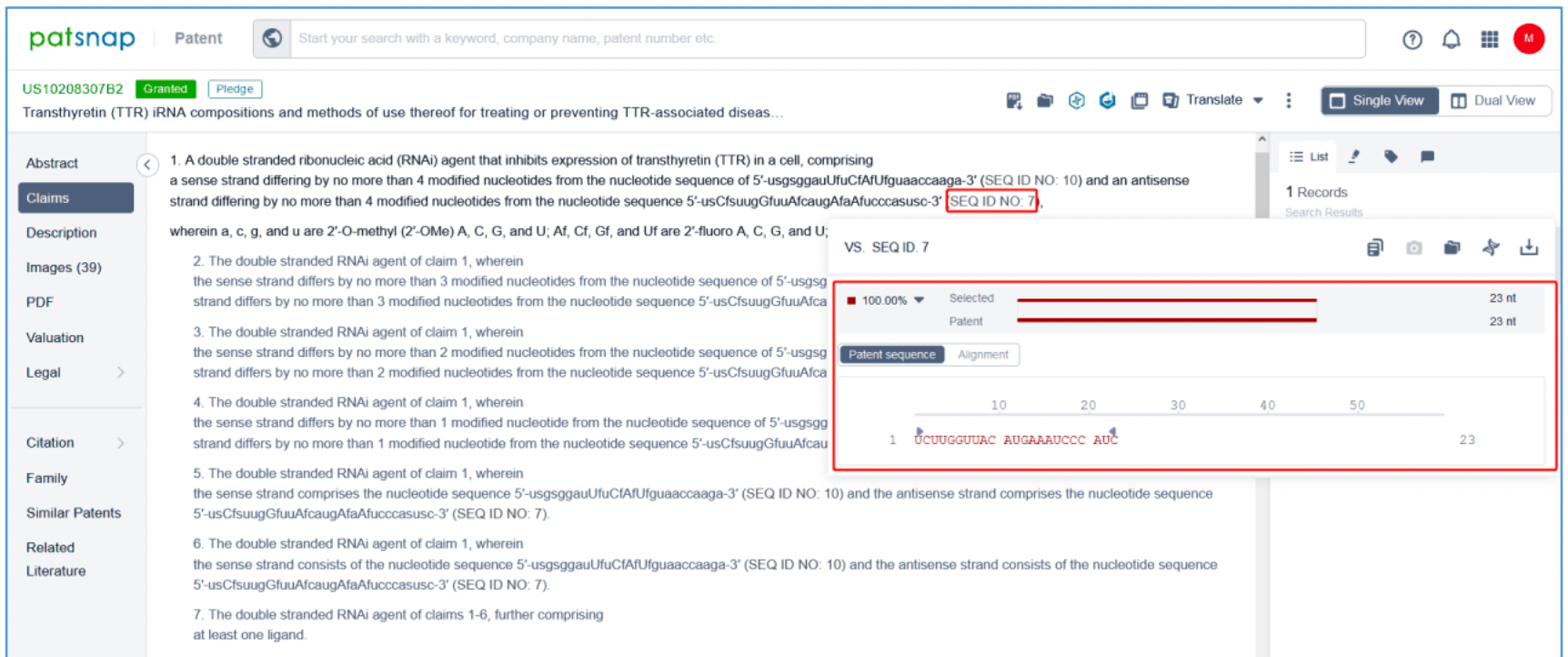
Independent claim 1 of US10208307B2, a family patent of PCT application US20170029817A1, states: "A double stranded ribonucleic acid (RNAi) agent that inhibits expression of transthyretin (TTR) in a cell, comprising a sense strand differing by no more than 4 modified nucleotides from the nucleotide sequence of 5' - usgsggauUfuCfAfUfguaaccaaga-3' (SEQ ID NO: 10) and an antisense strand differing by no more than 4 modified nucleotides from the nucleotide sequence 5' - usCfsuugGfuuAfcaugAfaAfucccasusc-3' (SEQ ID NO: 7), wherein a, c, g, and u are 2′-O-methyl (2′-OMe) A, C, G, and U; Af, Cf, Gf, and Uf are 2′-fluoro A, C, G, and U; and s is a phosphorothioate linkage. Hover over SEQ ID NO: 10 and SEQ ID NO: 7 in claim 1 to display the sense strand sequence, UGGGAUUUCAUGUAACCAAGA (SEQ ID NO: 10) and the antisense strand sequence, UCUUGGUUACAUGAAAUCCCAUC (SEQ ID NO: 7). These sequences have a 100% match with the vutrisiran sequence disclosed in Synapse. Independent claim 1 further specifies the modification of the sense strand and this antisense strand.
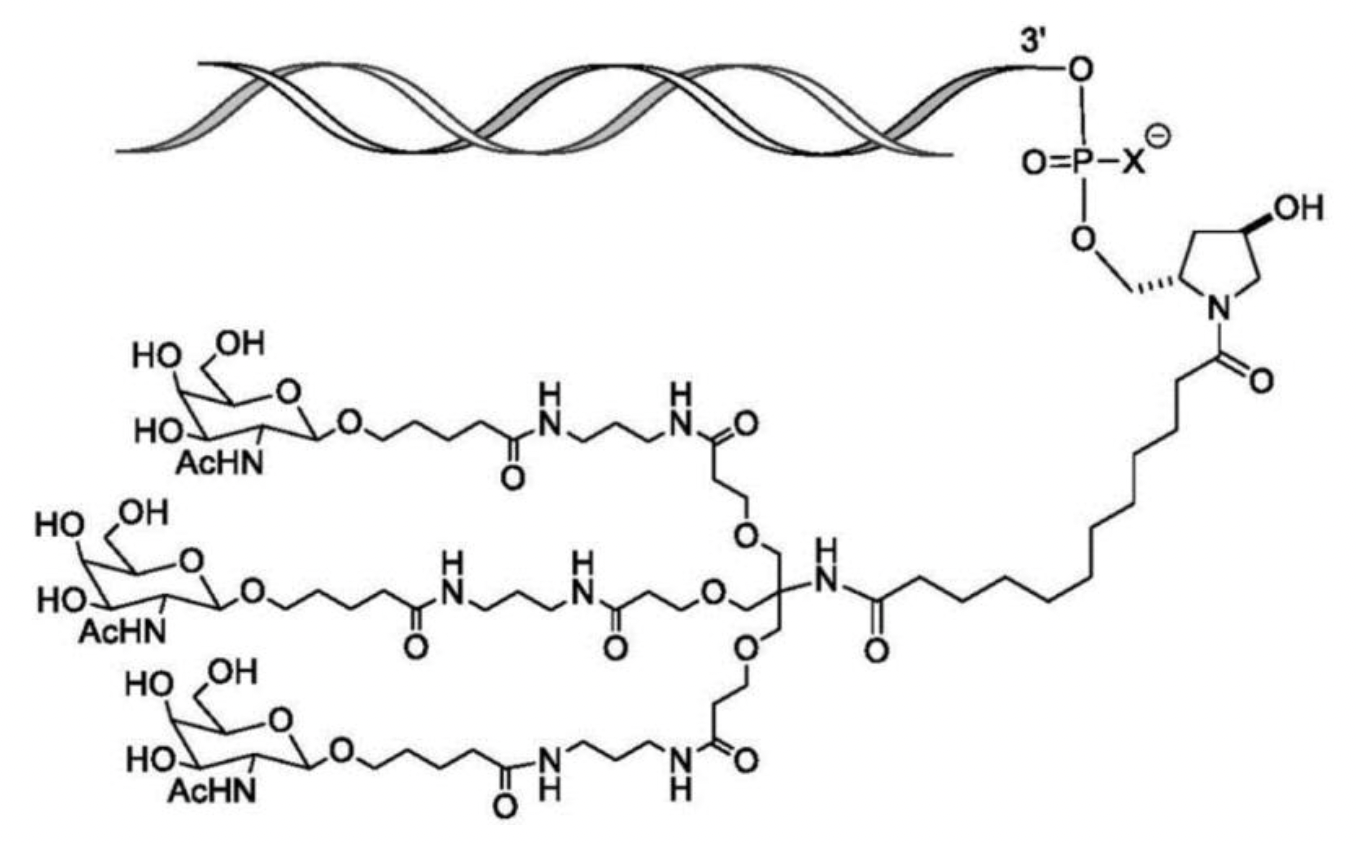
Furthermore, US10208307B2 also discloses a ligand structure conjugated to the sense strand of “one or more GalNAc derivatives attached through a bivalent or trivalent branched linker.”
For more information, you can click the following article link:
siRNA Drug Amvuttra Patent Research and Practical Operation Guide(3)
For more information, please click the image link below to access the full report.