EU Approves BeiGene's TEVIMBRA for First-Line Treatment of Advanced Esophageal and Gastric Cancers
BeiGene, Ltd., a worldwide oncology firm planning to rebrand as BeOne Medicines, has announced that the European Commission granted approval for TEVIMBRA® (tislelizumab) to be used alongside chemotherapy as a first-line therapy for esophageal squamous cell carcinoma (ESCC) and gastric or gastroesophageal junction (G/GEJ) adenocarcinoma.
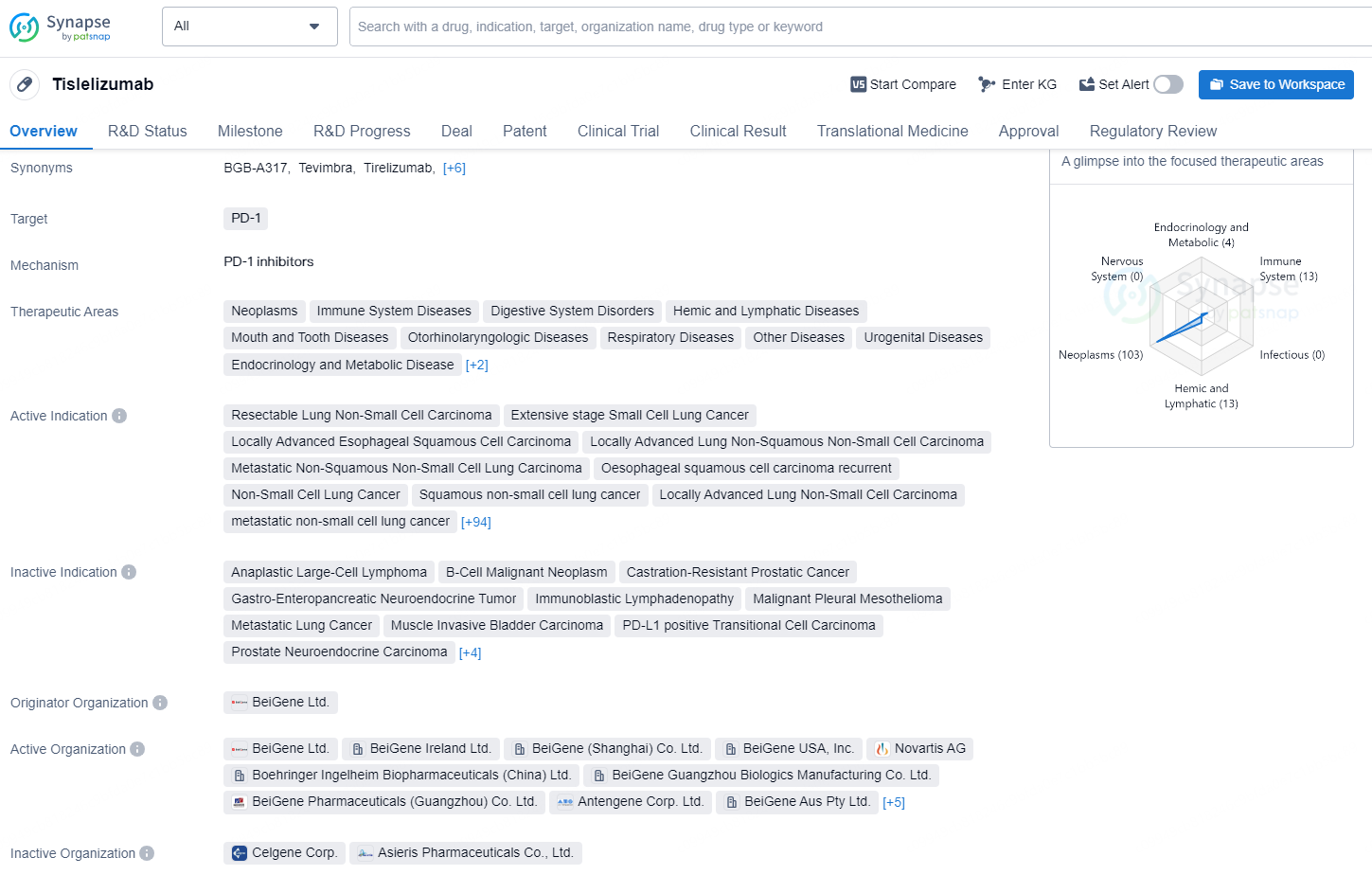
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
“Patients who are diagnosed with advanced gastric and esophageal cancers typically face median survival durations that span several months rather than years, which emphasizes the critical need for more efficient treatment alternatives,”remarked Prof. Florian Lordick, the Director and Professor of Oncology at the University Cancer Center Leipzig in Germany. “The strong findings from the RATIONALE-305 and 306 studies highlight the distinctive clinical characteristics of tislelizumab, showing its potential to significantly enhance outcomes for suitable patients, thus providing new optimism in areas where it is most desperately required.”
For esophageal squamous cell carcinoma (ESCC), the newly expanded indication permits the use of TEVIMBRA combined with platinum-based chemotherapy as a first-line option for adult patients with unresectable, locally advanced, or metastatic cancers that exhibit PD-L1 expression with a tumor area positivity (TAP) score of 5% or higher. In the case of gastric or gastroesophageal junction (G/GEJ) adenocarcinoma, TEVIMBRA's expanded indication allows for its use with both platinum-based and fluoropyrimidine-based chemotherapy for adult patients suffering from HER2-negative, locally advanced unresectable, or metastatic conditions, whose tumors also demonstrate PD-L1 with a TAP score of at least 5%.
“TEVIMBRA acts as the fundamental agent in our solid tumor portfolio, which is pivotal to BeiGene's dedication to offering groundbreaking therapies to a larger cohort of cancer patients, with over 1.3 million individuals having been treated globally with this medication,” stated Mark Lanasa, M.D., Ph.D., Chief Medical Officer for Solid Tumors at BeiGene. “Within a little over one year, we have secured approvals across six indications in the European Union, and we are eager to work towards ensuring that patients throughout Europe have timely and extensive access to TEVIMBRA.”
The application for expanded indication in first-line ESCC was grounded on results from BeiGene’s RATIONALE-306 (NCT03783442), a randomized, placebo-controlled, double-blind, global Phase 3 trial aimed at assessing the efficacy and safety of TEVIMBRA when combined with chemotherapy for the first-line management of patients with unresectable, locally advanced recurrent, or metastatic ESCC. The trial involved 649 participants from research institutions throughout Europe, North America, and the Asia-Pacific region. It successfully achieved its primary endpoint; first-line administration of TEVIMBRA alongside chemotherapy yielded a statistically significant and clinically relevant overall survival (OS) benefit in comparison to placebo plus chemotherapy within the intent-to-treat cohort. The median OS was recorded at 17.2 months for the TEVIMBRA group combined with chemotherapy, contrasted with 10.6 months for the placebo plus chemotherapy cohort (HR: 0.66 [95% CI, 0.54-0.80, one-sided p-value of < 0.0001]), indicating a 34% decrease in mortality risk. The three-year OS rate in the PD-L1≥5% population also showed significant improvement in favor of the TEVIMBRA group (median 19.1 months vs. 10.0 months, respectively; HR: 0.62 [95% CI, 0.49-0.79]), demonstrating a 38% reduction in death risk.
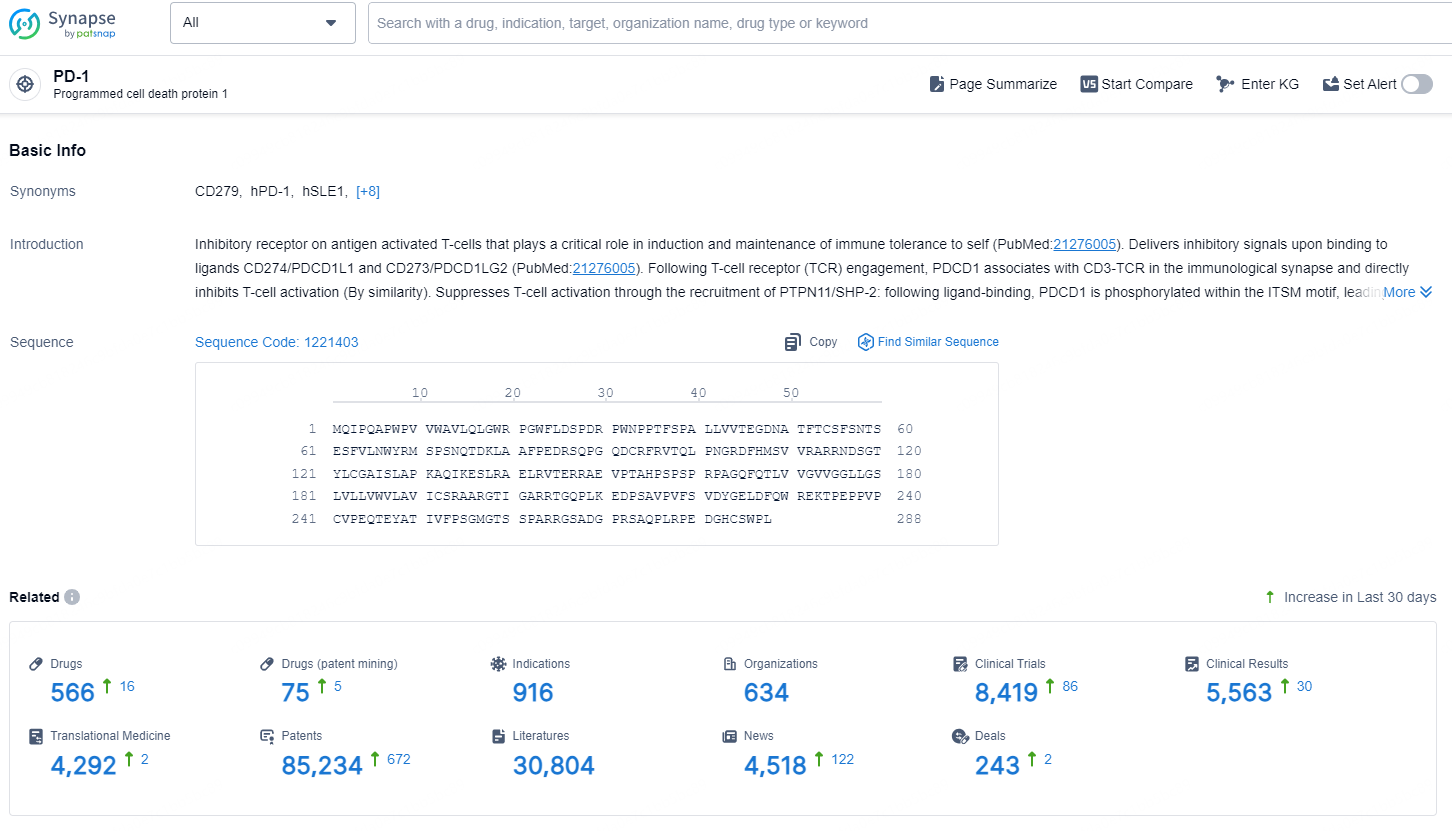
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of December 2, 2024, there are 566 investigational drugs for the PD-1 target, including 916 indications, 634 R&D institutions involved, with related clinical trials reaching 8419, and as many as 85234 patents.
Tislelizumab is a monoclonal antibody drug that targets PD-1 and is used in the treatment of a wide range of therapeutic areas including neoplasms, immune system diseases, digestive system disorders, hemic and lymphatic diseases, mouth and tooth diseases, otorhinolaryngologic diseases, respiratory diseases, urogenital diseases, endocrinology and metabolic disease, eye diseases, skin and musculoskeletal diseases.