Menarini Group Highlights ORSERDU® (Elacestrant) Findings at 2024 San Antonio Breast Cancer Symposium
The Menarini Group, an international leader in pharmaceuticals and diagnostics, along with its wholly-owned subsidiary Stemline Therapeutics, Inc., dedicated to providing innovative oncology solutions for cancer patients, is set to unveil new and enhanced findings regarding ORSERDU® (elacestrant) at the forthcoming 2024 San Antonio Breast Cancer Symposium (SABCS), scheduled for December 10-13, 2024. A key highlight will be the presentation of real-world progression-free survival (rwPFS) data for ORSERDU in adult patients diagnosed with ER+/HER2-, advanced, or metastatic breast cancer (mBC). The company will also share updated efficacy outcomes for the combination of elacestrant and abemaciclib, in addition to a comprehensive safety assessment derived from the phase 1b/2 studies of both ELECTRA and ELEVATE trials.
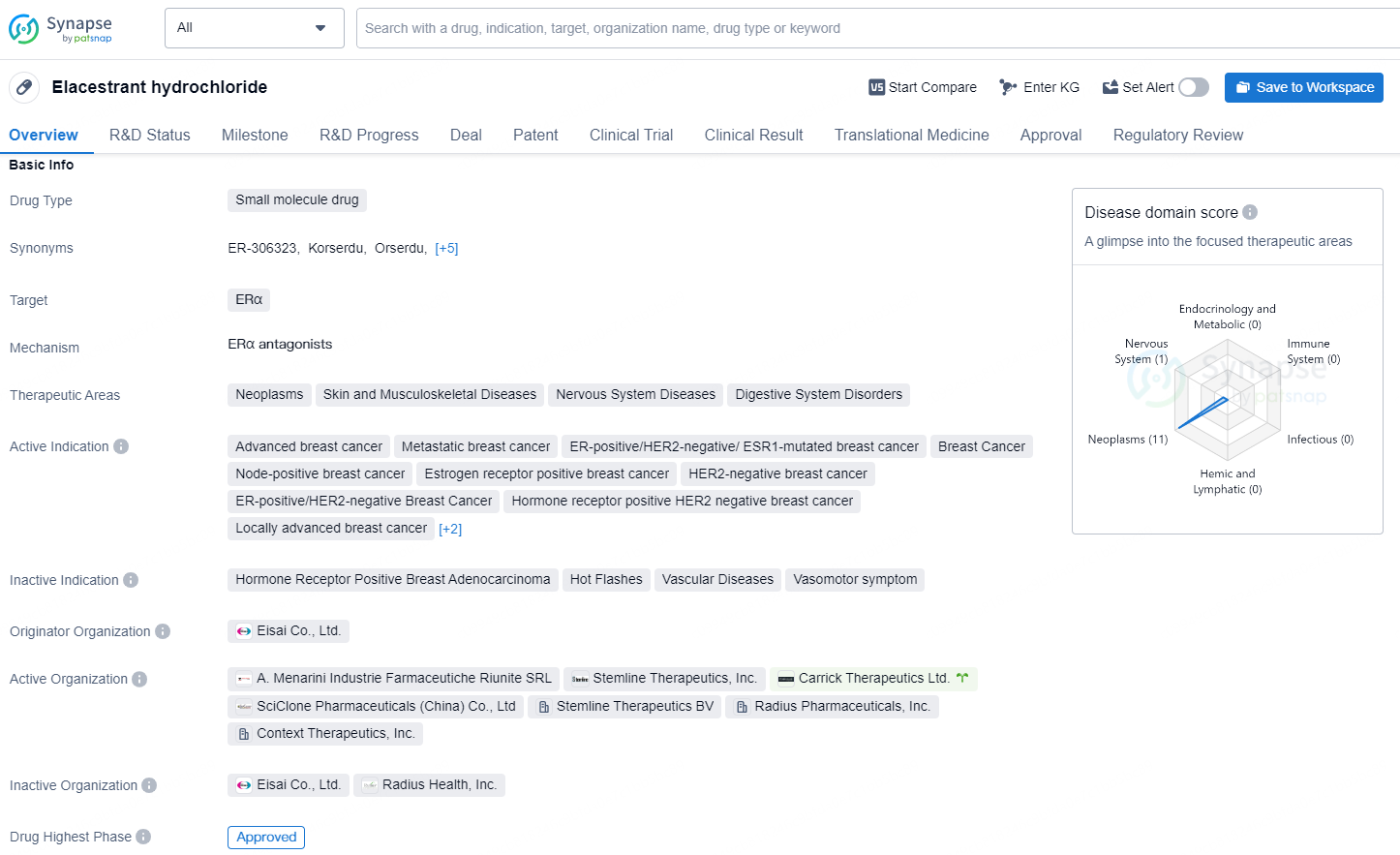
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
ORSERDU is the first and only oral selective estrogen receptor degrader (SERD) that has been authorized for the treatment of tumors with ESR1 mutations, commonly found in up to 50% of ER+, HER2- advanced or metastatic breast cancer (mBC) cases due to previous endocrine therapy exposure in a metastatic context. Since receiving approval from the U.S. Food & Drug Administration (FDA) in January 2023, ample time has elapsed to assess the application of ORSERDU in the current landscape of mBC treatment.
Findings to be unveiled at SABCS 2024 will highlight the efficacy of ORSERDU in real-world scenarios involving patients with ER+/HER2- advanced or mBC. The data for the overall population indicated a median real-world progression-free survival (rwPFS) of 6.8 months, while patients who had received 1-2 lines of prior endocrine therapy in mBC experienced a median rwPFS of 8 months. The rwPFS observed remains consistent across various subgroups analyzed. Further updated results and additional insights regarding other patient categories will be shared during the congress.
"These promising findings demonstrate significant real-world progression-free survival with ORSERDU as a standalone treatment," stated Virginia Kaklamani, MD, DSc, a breast medical oncologist and professor at UT Health San Antonio, MD Anderson Cancer Center. "As a clinician, this data emphasizes the importance of evaluating tumors for the ESR1 mutation at each stage of disease progression through liquid biopsy, enabling us to tailor treatments effectively and enhance patient care."
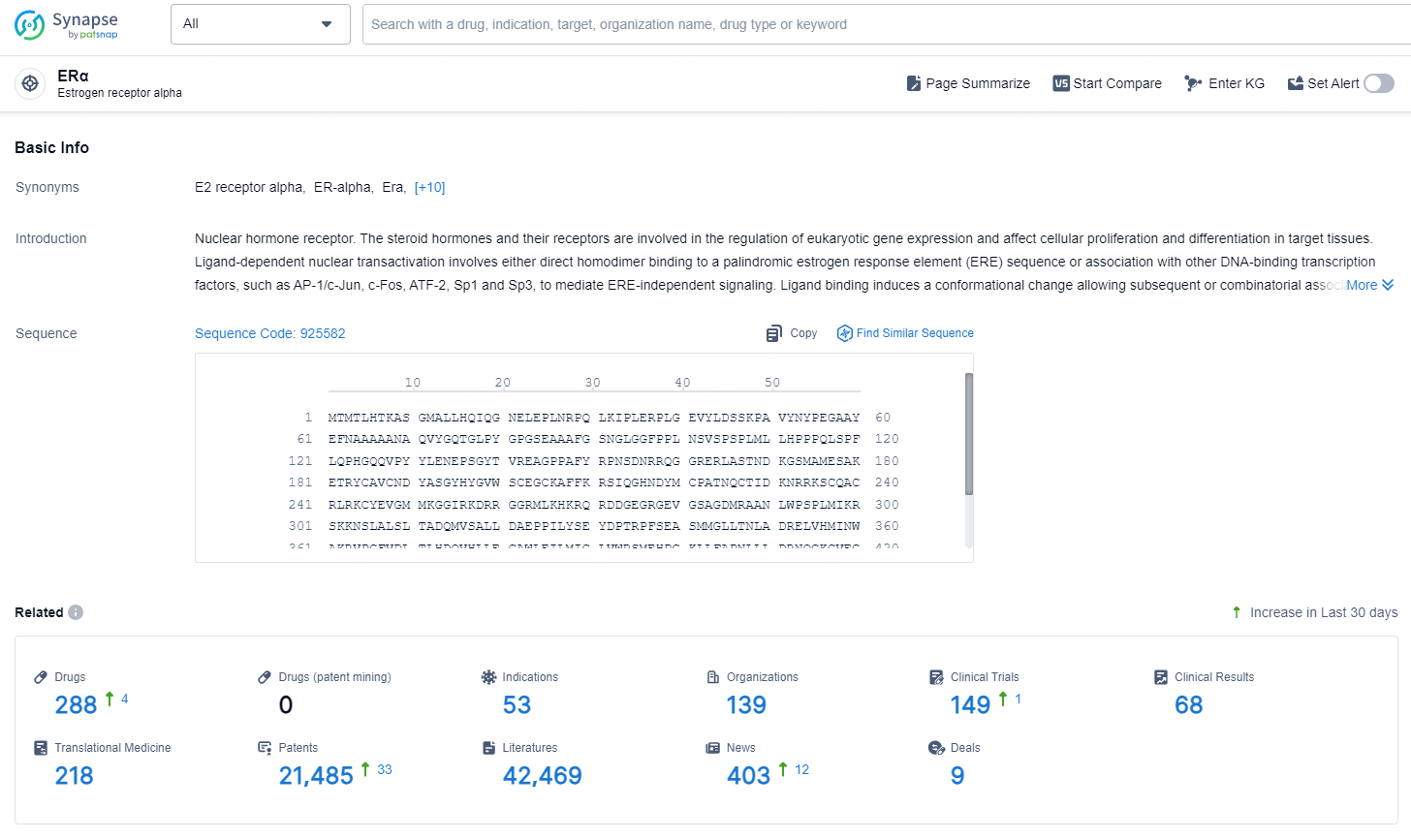
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of November 28, 2024, there are 288 investigational drugs for the ERα target, including 53 indications, 139 R&D institutions involved, with related clinical trials reaching 149, and as many as 21485 patents.
Elacestrant hydrochloride is a small molecule drug designed to target ERα, and it is used to treat various therapeutic areas including neoplasms, skin and musculoskeletal diseases, nervous system diseases, and digestive system disorders.