EU Commission Approves Astellas' VYLOY™ for Advanced Stomach and GEJ Cancer Treatment
Astellas Pharma Inc. (TSE: 4503, President and CEO: Naoki Okamura, "Astellas") announced that the European Commission (EC) has granted approval for VYLOY™ (zolbetuximab) to be used alongside fluoropyrimidine- and platinum-based chemotherapy for the initial treatment of adult patients with locally advanced, unresectable, or metastatic HER2-negative gastric or gastroesophageal junction (GEJ) adenocarcinoma, specifically for tumors that are positive for claudin (CLDN) 18.2. The European Medicines Agency has advised keeping zolbetuximab's orphan medicinal product status due to the generally poor survival rates seen in gastric and GEJ cancers.
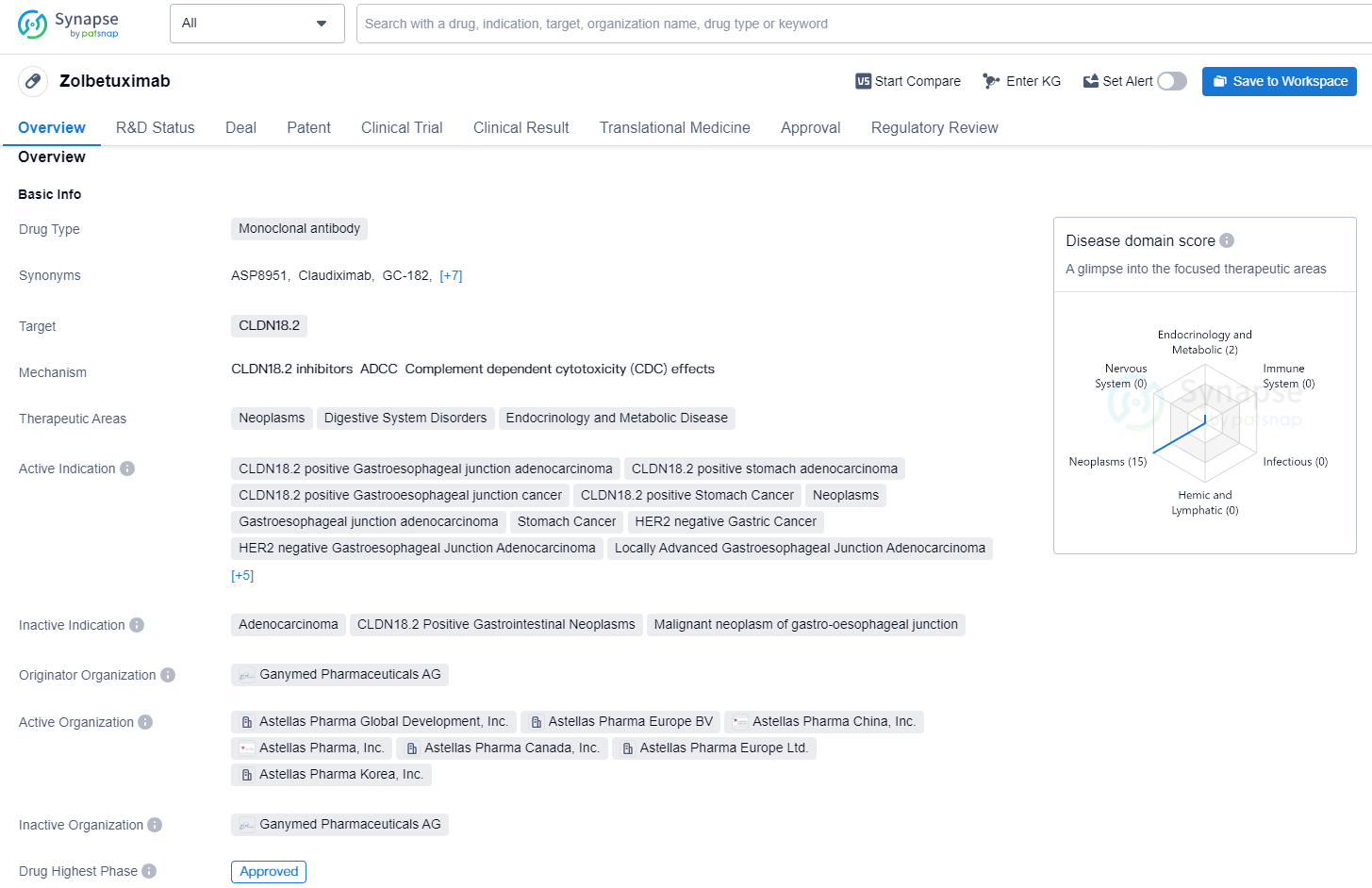
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Zolbetuximab represents the inaugural and sole approved monoclonal antibody specifically engineered to target gastric tumor cells expressing the CLDN18.2 biomarker, thereby enabling a more individualized cancer treatment approach. In Phase 3 clinical trials for zolbetuximab, around 38% of adult patients with advanced and metastatic gastric and GEJ cancers exhibited CLDN18.2 positive tumors.1,2 By binding to CLDN18.2 on tumor cell membranes, zolbetuximab induces antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity, and suppression of tumor growth.3
Zorana Maravic, the Chief Executive Officer of Digestive Cancers Europe (DiCE), stated:
“Unfortunately, gastric and gastroesophageal junction cancers are frequently diagnosed at advanced or metastatic stages due to their symptoms being similar to more common gastric conditions. Historically, treatment options at these stages have been rather limited. Prompt diagnosis followed by personalized treatment and care is crucial to improving patient survival rates and quality of life.”
Moitreyee Chatterjee-Kishore, Ph.D., M.B.A., Senior Vice President and Head of Immuno-Oncology Development at Astellas, commented:
“We are thrilled to introduce zolbetuximab, a pioneering targeted treatment, to patients in Europe, where gastric and gastroesophageal cancers rank as the sixth leading cause of cancer-related fatalities. Zolbetuximab marks the advent of a new era in precision oncology for these advanced cancers, highlighting our unwavering dedication to advancing scientific innovation to improve patient outcomes.”
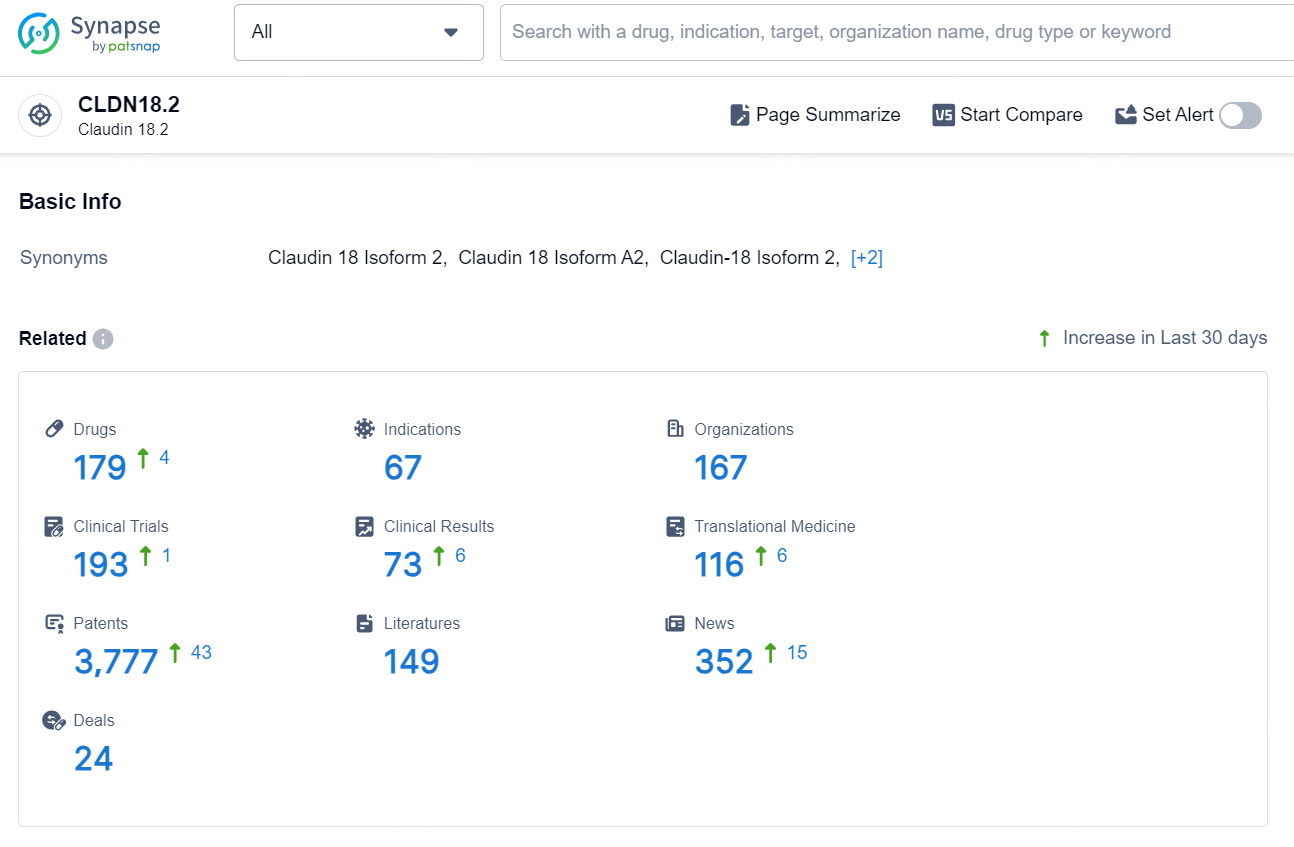
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 24, 2024, there are 179 investigational drugs for the CLDN18.2 targets, including 67 indications, 167 R&D institution involved, with related clinical trial reaching 193, and as many as 3777 patents.
Zolbetuximab is a monoclonal antibody drug that targets CLDN18.2 and is indicated for the treatment of various neoplasms and digestive system disorders. It is also used in the management of endocrinology and metabolic diseases. The drug is specifically indicated for CLDN18.2 positive gastroesophageal junction adenocarcinoma, stomach adenocarcinoma, gastroesophageal junction cancer, stomach cancer, pancreatic adenocarcinoma, and HER2 negative gastric cancer. It is also approved for locally advanced and metastatic gastroesophageal junction adenocarcinoma, as well as locally advanced gastric adenocarcinoma.