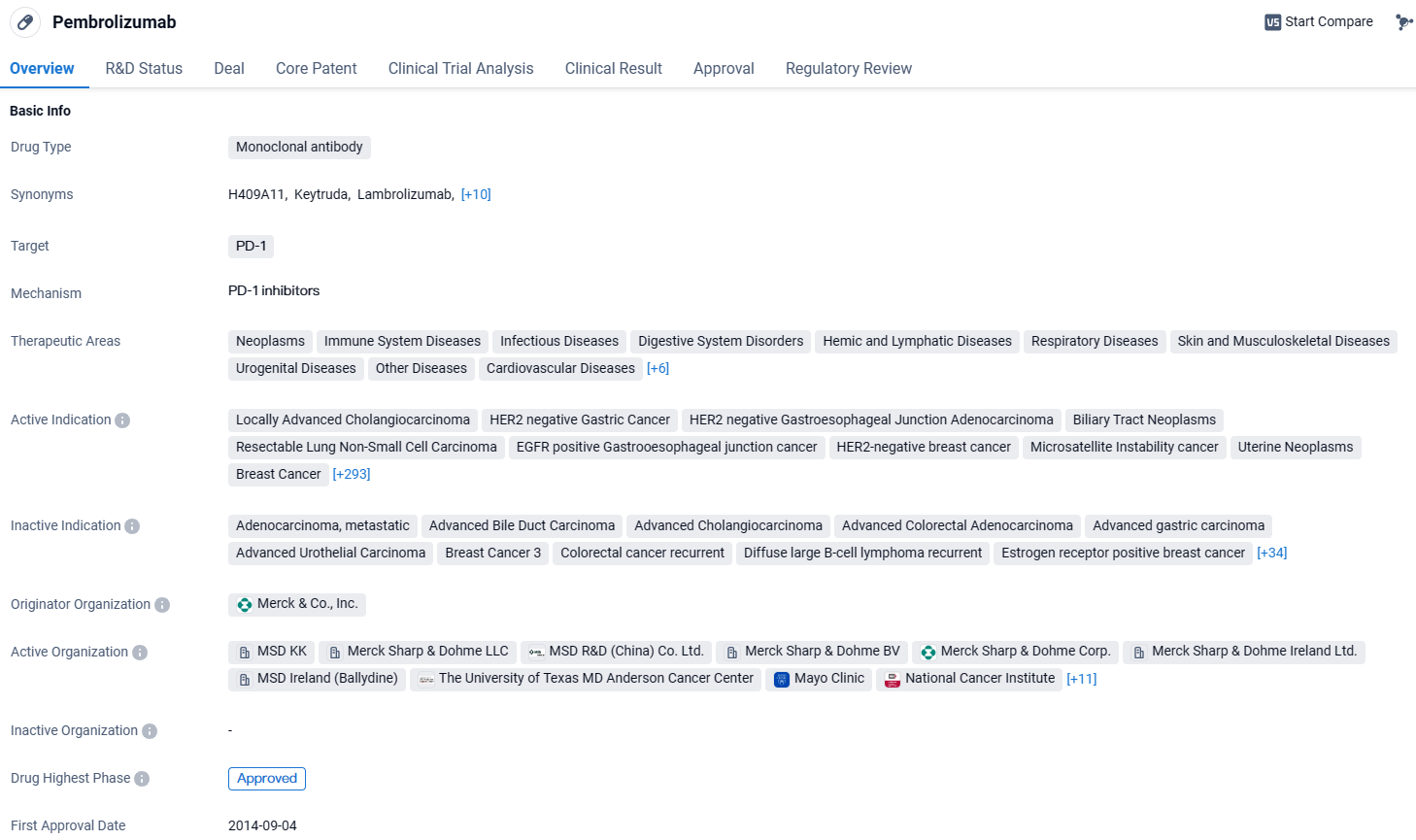
EU Approves Merck's KEYTRUDA® for Initial and Adjuvant Treatment in High-Risk NSCLC
Merck, made a public declaration concerning the authorization by the European Union's governing body for its drug, KEYTRUDA. This immunotherapy, which targets the programmed cell death protein 1 (PD-1), has been sanctioned for use initially with a regimen of chemotherapy agents that include platinum for early-stage intervention, followed by its solitary application for additional therapy in adult patients undergoing surgery for non-small cell lung cancer with a significant likelihood of returning.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The European Commission has granted authorization subsequent to a favorable evaluation by the Committee for Medicinal Products for Human Use in February 2024, which was contingent on outcomes from the KEYNOTE-671 Phase 3 study.
Over a median observation period of nearly 30 months, the combination of KEYTRUDA and chemotherapy as preoperative treatment, followed by sole use of KEYTRUDA post-surgery, considerably boosted survival rates. It demonstrated a 28% decrease in mortality risk among patients with operable stage II, IIIA, or IIIB NSCLC compared to the standard procedure of placebo plus chemotherapy followed by placebo after surgical removal, independent of PD-L1 expression levels.
"The statistics for lung cancer as the foremost cause of cancer-related mortality in Europe remain unyielding, underscoring the critical necessity to intervene at more preliminary phases of the illness. Here, we hope to enact the greatest change," reported Dr. Solange Peters, who heads up the oncology unit specializing in thoracic malignancies at the Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland.
With the recent authorization, the KEYTRUDA treatment regimen can now be marketed across the 27 European Union countries, including Iceland, Liechtenstein, Norway, and Northern Ireland. KEYTRUDA boasts approvals for a total of six NSCLC treatments and has 27 separate indications authorized across the EU. In the United States, KEYTRUDA received the nod in October 2023 to treat operable NSCLC, with it being used in combination with platinum-based chemotherapy before surgery and continued as a standalone therapy post-operatively.
"The sanction of our pioneering anti-PD-1/L1 therapy for both pre- and post-operative neoadjuvant use in resectable NSCLC, premised on superior survival benefits, signifies our persistent strides in enhancing early-stage lung cancer therapy," expressed Marjorie Green, the head of global clinical oncology development at Merck Research Laboratories and senior vice president.
She continued, "We look forward to maintaining this trajectory as we aim for further global regulatory endorsements for this regimen, and as we join forces with the medical community to catalyze early detection of lung cancer—an imperative objective."
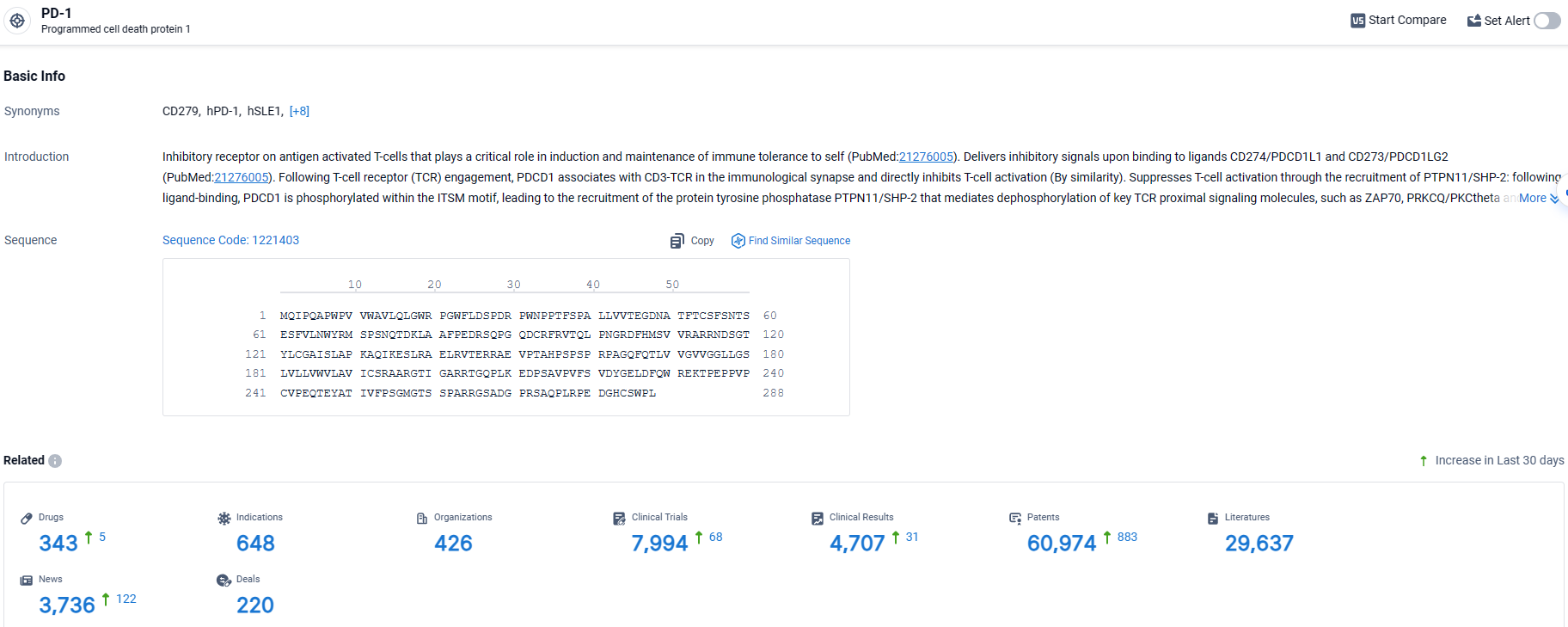
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 2, 2024, there are 343 investigational drugs for the PD-1 target, including 648 indications, 426 R&D institutions involved, with related clinical trials reaching 7994, and as many as 60974 patents.
pembrolizumab is a monoclonal antibody drug that targets PD-1 and has been approved for the treatment of various cancers and other diseases. It has shown promising results in clinical trials and has received regulatory approvals globally. The drug has been granted several regulatory designations to facilitate its development and availability to patients in need.