EU Approves Pfizer's HYMPAVZI™ for Severe Hemophilia A or B Treatment
Pfizer Inc. (NYSE: PFE) has disclosed that the European Commission (EC) has approved marketing authorization for HYMPAVZI™ (marstacimab) intended for the regular prevention of bleeding episodes in patients aged 12 and older who weigh a minimum of 35 kg and have severe hemophilia A (congenital factor VIII [FVIII] deficiency, FVIII <1%) without the presence of FVIII inhibitors, or severe hemophilia B (congenital factor IX [FIX] deficiency, FIX <1%) without FIX inhibitors.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
HYMPAVZI is the inaugural and sole anti-tissue factor pathway inhibitor (anti-TFPI) authorized in the European Union (EU) for the treatment of hemophilia A and B. It is also the first hemophilia therapy in the EU that can be administered via a pre-filled, auto-injector pen. This treatment provides a subcutaneous administration option with a weekly dosing regimen and requires minimal preparation for each use.
“There is a significant burden associated with the current standard care for hemophilia A and B, which includes the labor-intensive preparation and administration of infusions and injections, leading to possible missed doses and a higher risk of bleeding,” commented Dr. Laurent Frenzel, Head of the Hemophilia Treatment and Research Center at Necker-Enfants Malades Hospital in Paris. “HYMPAVZI represents a major advancement for qualifying patients, potentially offering effective bleed prevention along with weekly subcutaneous delivery via a pre-filled injector.”
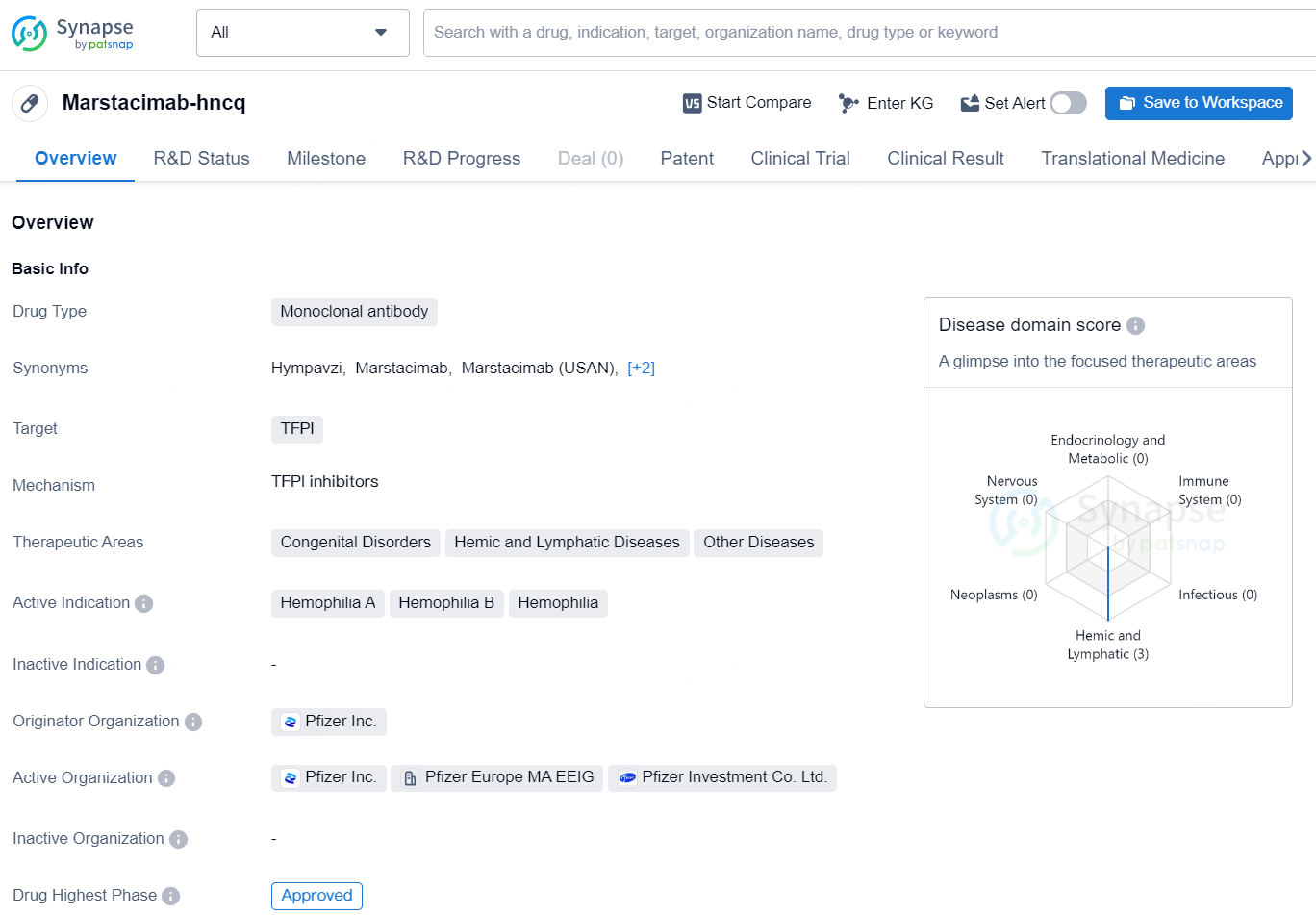
Hemophilia refers to a group of rare genetic bleeding disorders stemming from a deficiency in clotting factors (FVIII in hemophilia A and FIX in hemophilia B), affecting over 800,000 individuals worldwide. Diagnosed typically in early childhood, hemophilia hinders the blood's clotting ability, raising the risk of frequent internal bleeding in joints, which can result in permanent joint damage. Despite notable advancements in treatment options over the years, many individuals with hemophilia still face bleeding incidents and often require recurring intravenous infusions multiple times each week.
“HYMPAVZI presents a pioneering treatment choice for those living with hemophilia, a condition that frequently leads to ongoing joint bleeds and can interfere with everyday activities as simple as climbing stairs,” stated Alexandre de Germay, Chief International Commercial Officer and Executive Vice President at Pfizer. “This approval builds on Pfizer’s longstanding commitment of over 40 years to enhance care standards in hemophilia, and we are eager to provide this treatment, which reduces bleeding compared to factor prophylaxis and requires minimal preparation, addressing an essential need for eligible patients.”
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
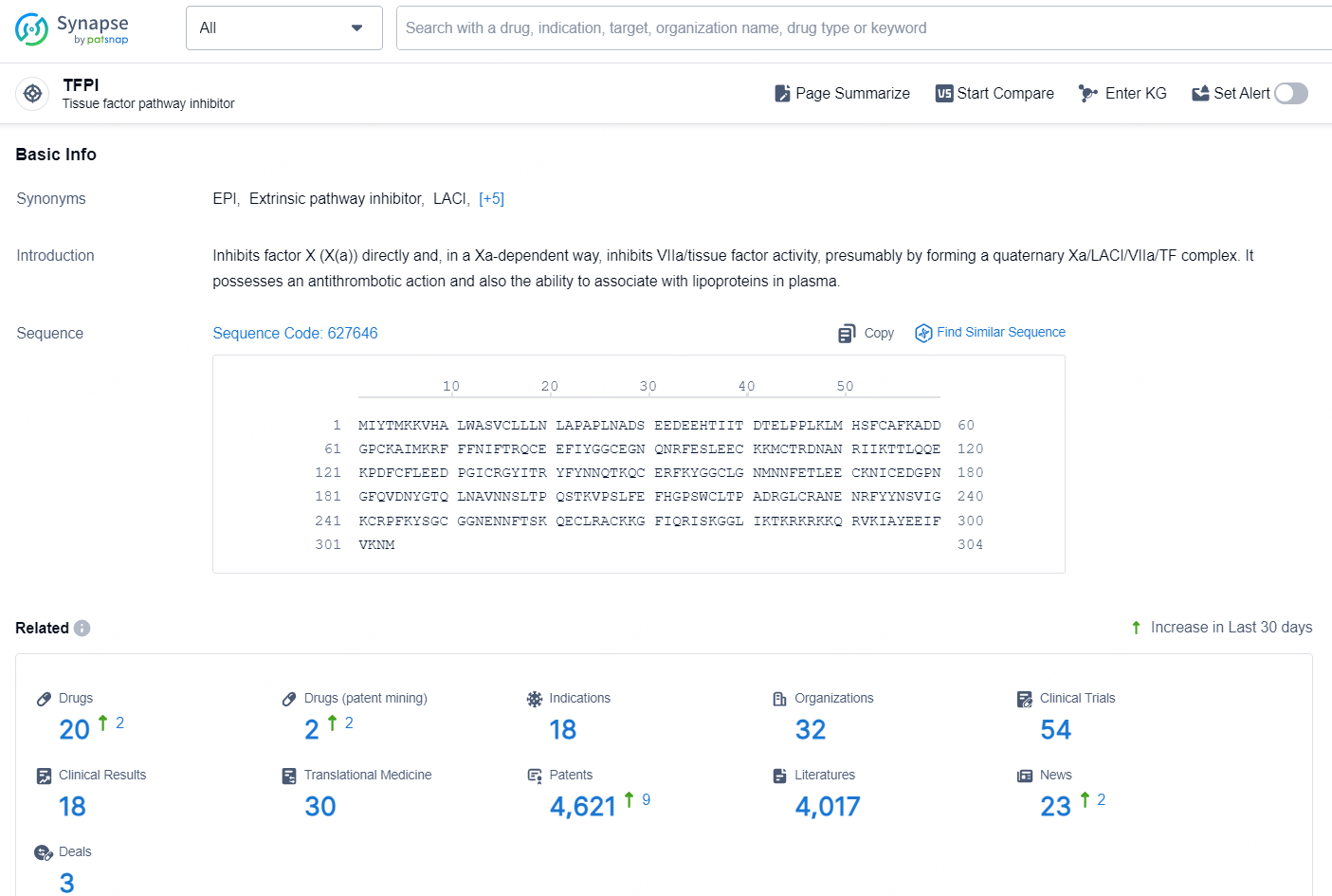
According to the data provided by the Synapse Database, As of November 26, 2024, there are 20 investigational drugs for the TFPI target, including 18 indications, 32 R&D institutions involved, with related clinical trials reaching 54, and as many as 4621 patents.
Marstacimab-hncq is a monoclonal antibody drug developed by Pfizer Inc. and is designed to target Tissue Factor Pathway Inhibitor (TFPI). This drug has been indicated for the treatment of various congenital disorders, hemic and lymphatic diseases, as well as other diseases related to hemophilia A, hemophilia B, and hemophilia.