Lilly's muvalaplin reduced lipoprotein(a) levels in adults at high risk
Eli Lilly and Company (NYSE: LLY) has announced encouraging Phase 2 findings for muvalaplin, a candidate drug that is taken orally once a day, selectively inhibiting lipoprotein(a) [Lp(a)], which is a genetically linked risk factor for heart disease. The trial showed that muvalaplin effectively lowered increased Lp(a) levels in adult participants, achieving its main objective of the percentage change in Lp(a) from the baseline by week 12.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
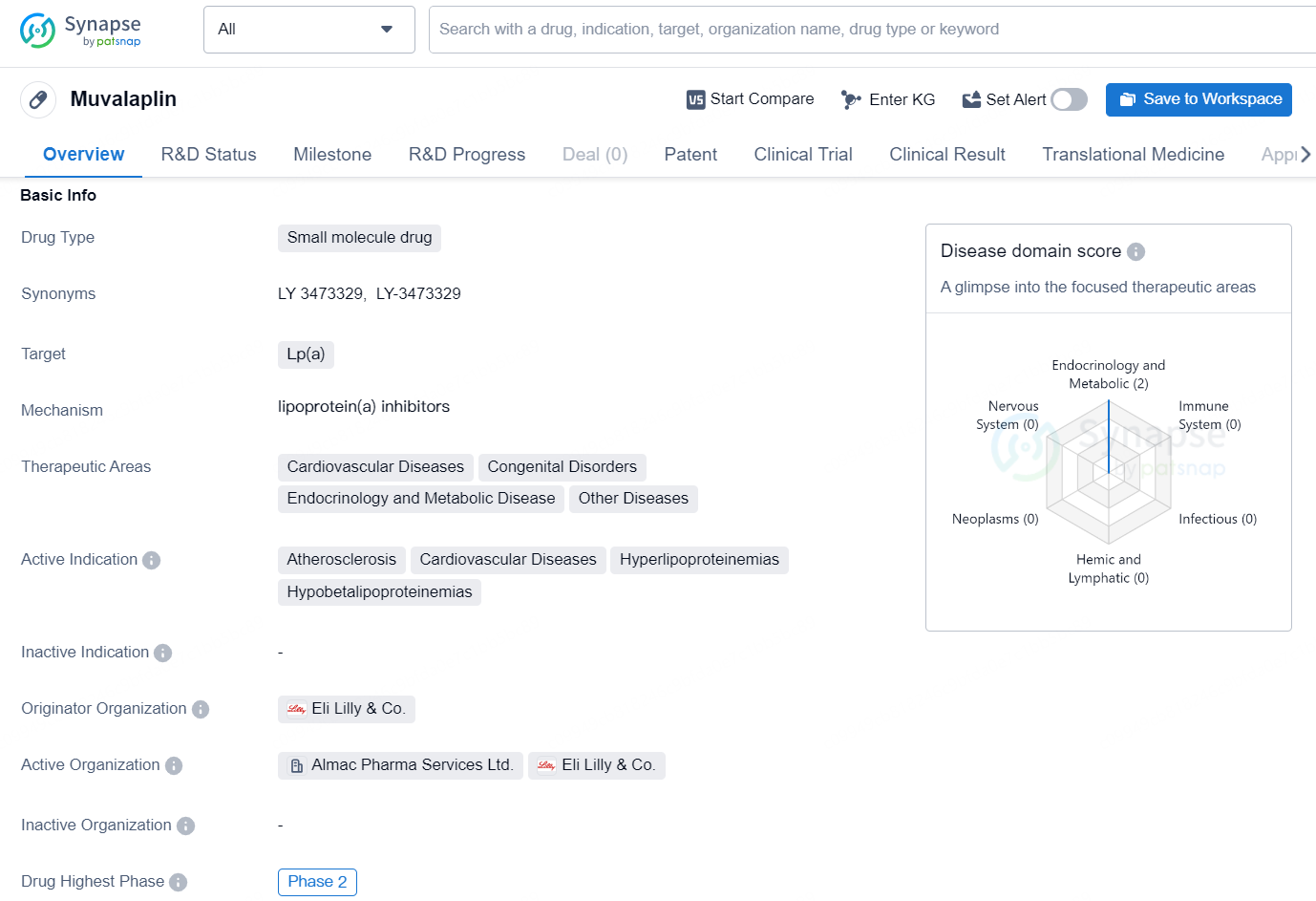
At the 12-week primary endpoint, treatment with muvalaplin at doses of 10 mg, 60 mg, and 240 mg resulted in significant decreases in Lp(a) levels when compared to a placebo. The reductions adjusted for placebo reached as high as 85.8% with an intact Lp(a) assay and 70.0% with an apo(a) assay. The specific reductions were recorded as 47.6% for the 10 mg dose, 81.7% for the 60 mg dose, and 85.8% for the 240 mg dose using the intact Lp(a) assay. The apo(a) assay yielded reductions of 40.4% for the 10 mg dose, 70.0% for the 60 mg dose, and 68.9% for the 240 mg dose.
“Elevated Lp(a) levels have been identified as a major risk factor for atherosclerotic cardiovascular disease, impacting over one billion adults globally,” stated Stephen J. Nicholls, MBBS, Ph.D., director at the Victorian Heart Hospital and Institute and a professor of cardiology at Monash University in Australia. “Existing cholesterol-lowering treatments do not target Lp(a) levels, indicating a significant need for solutions for patients with cardiovascular conditions. These findings mark an important scientific breakthrough that might lower the risk of cardiovascular incidents, such as heart attacks and strokes, with a daily oral medication.”
Lilly is investigating muvalaplin, a highly effective small molecule that inhibits Lp(a) production by disrupting the initial binding between apolipoprotein(a) [apo(a)] and apolipoproteinB (apoB). Approximately 20% of the U.S. population, or around 63 million individuals, are reported to have elevated Lp(a) levels. High Lp(a) concentrations can significantly increase the likelihood of heart attacks and are linked to other cardiovascular complications.
“While injectable therapies for Lp(a) are currently undergoing Phase 3 trials, including Lilly's lepodisiran project, these results represent the first positive Phase 2 outcomes for an oral treatment,” commented Ruth Gimeno, Ph.D., group vice president for Diabetes and Metabolic Research at Lilly Research Laboratories. “We are excited by these promising results and anticipate further investigation into muvalaplin's next steps.”
Muvalaplin also achieved secondary endpoints across all three doses tested (10 mg, 60 mg, and 240 mg). Each of the doses demonstrated statistical significance in Lp(a) reductions, with the 60 mg and 240 mg doses also achieving significance in reducing apoB levels. Specific observations included:
Using the intact Lp(a) assay, 64.2% (10 mg), 95.9% (60 mg), and 96.7% (240 mg) of participants reached an Lp(a) level below 125 nmol/L by week 12, compared to 6.0% in the placebo cohort.
With the apo(a) assay, the percentages of participants achieving Lp(a) levels below 125 nmol/L were 38.9% (10 mg), 81.9% (60 mg), and 77.4% (240 mg), compared to just 3.6% in the placebo group.
ApoB levels decreased across all doses, with placebo-adjusted reductions of 8.9% (10 mg), 13.1% (60 mg), and 16.1% (240 mg).
The frequency of adverse events was comparable between muvalaplin and placebo cohorts. Adverse events tied to the study medication were noted in 14.9% of the placebo group, 5.9% of the 10 mg group, 14.3% of the 60 mg group, and 14.7% of the 240 mg group. The rate of adverse events leading to treatment discontinuation varied from 0% to 8.8% across different treatment groups, with occurrences being isolated incidents across various organ systems. There were no reported fatalities in the study.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
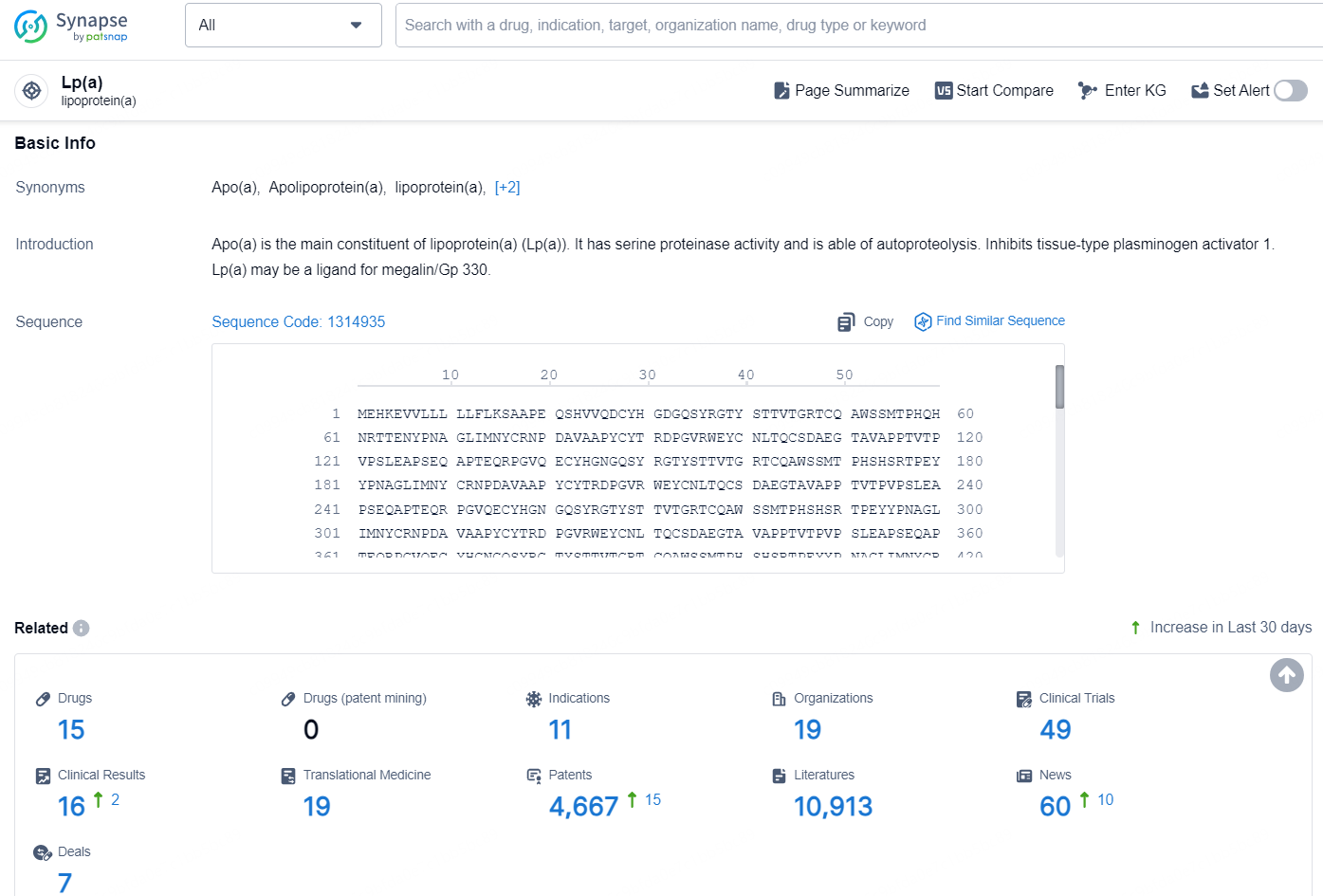
According to the data provided by the Synapse Database, As of November 26, 2024, there are 15 investigational drugs for the Lp(a) target, including 11 indications, 19 R&D institutions involved, with related clinical trials reaching 49, and as many as 4667 patents.
Muvalaplin is a small molecule drug developed by Eli Lilly & Co. that targets Lp(a). The drug is being researched for its potential use in treating various therapeutic areas, including cardiovascular diseases, congenital disorders, endocrinology and metabolic disease, and other diseases.