EU Commission Approves Celltrion’s SteQeyma®, a Stelara® Biosimilar, for Chronic Inflammatory Disorders
Celltrion reported that the European Commission (EC) has granted approval for SteQeyma® (CT-P43), a biosimilar to ustekinumab, which references Stelara®, for managing several chronic inflammatory conditions. SteQeyma has received approval as a biologic treatment for conditions in the fields of gastroenterology, dermatology, and rheumatology. Stelara became the pioneering biologic therapy for Crohn’s disease targeting interleukin IL-12 and IL-23 cytokines, which are essential in inflammatory and immune responses.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The European Commission (EC) decided to approve SteQeyma in June 2024, following a favorable recommendation from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA). This approval of SteQeyma by the EC was grounded on comprehensive evidence, including outcomes from a Phase III trial involving adults with moderate to severe plaque psoriasis. The study's chief endpoint examined the change rate in the Psoriasis Area and Severity Index (PASI) for skin symptoms. Clinical findings indicated that SteQeyma is highly comparable to its reference product, Stelara, displaying no clinically significant differences in efficacy and safety.
“The EC approval of SteQeyma provides a vital new treatment option for patients. We are eager to introduce this innovative therapy, which has proven success in treating Crohn’s and other immune conditions,” stated Taehun Ha, Senior Vice President and Head of Europe Division at Celltrion. “Along with the approvals of Remsima SC and Yuflyma, this milestone plays a crucial role in our plan to enhance Celltrion’s immunology offerings. With Omlyclo's recent approval in May, we anticipate broadening our dermatology portfolio. This approval underscores our relentless commitment to expanding access to affordable, high-quality biologic medicines for patients.”
SteQeyma marks the seventh biosimilar by Celltrion cleared for use within the European Union (EU). It joins the ranks of Celltrion’s notable portfolio, including Remsima® SC, a subcutaneous version of infliximab approved in the EU, and other biosimilars such as Remsima® (biosimilar infliximab), Truxima® (biosimilar rituximab), Herzuma® (biosimilar trastuzumab), Yuflyma® (biosimilar adalimumab), Vegzelma® (biosimilar bevacizumab), and Omlyclo® (biosimilar omalizumab).
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
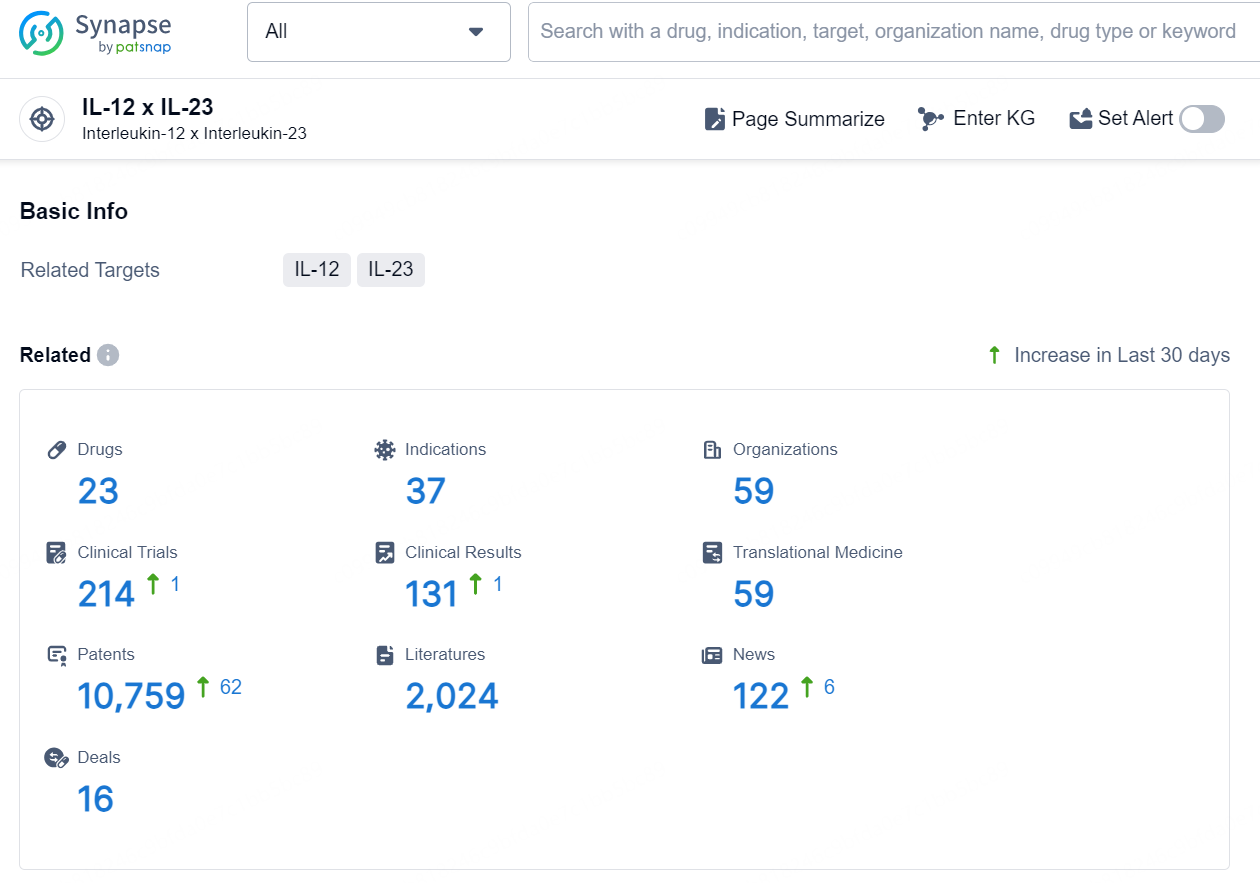
According to the data provided by the Synapse Database, As of August 27, 2024, there are 10 investigational drugs for the IL-12/IL-23 targets, including 172 indications, 42 R&D institutions involved, with related clinical trials reaching 748, and as many as 2518 patents.
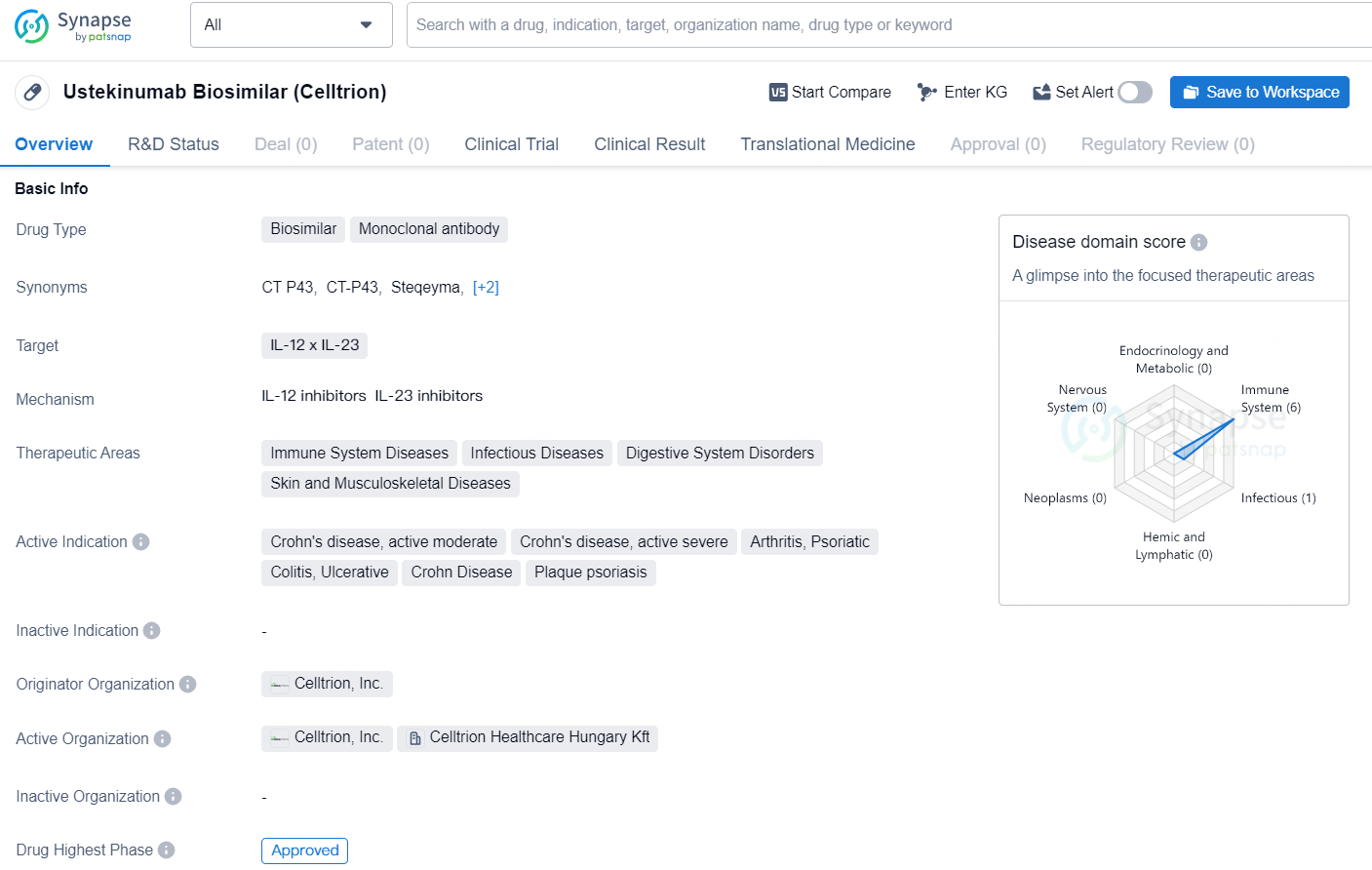
Ustekinumab Biosimilar (Celltrion) is a biosimilar drug classified as a monoclonal antibody. It is designed to target IL-12 and IL-23 and is intended to treat a range of therapeutic areas including immune system diseases, infectious diseases, digestive system disorders, and skin and musculoskeletal diseases. The drug is indicated for the treatment of Crohn's disease (active moderate and severe), psoriatic arthritis, ulcerative colitis, and plaque psoriasis.