Moderna Gains European Commission Nod for RSV Vaccine mRESVIA(R)
Moderna, Inc. (NASDAQ:MRNA) reported that the European Commission (EC) has approved mRESVIA® (mRNA-1345), an mRNA vaccine targeting respiratory syncytial virus (RSV), for marketing. This vaccine is designed to protect individuals aged 60 and above from lower respiratory tract disease caused by RSV. The marketing approval comes after a Positive Opinion from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA). This authorization is applicable across all 27 EU countries, along with Iceland, Liechtenstein, and Norway.
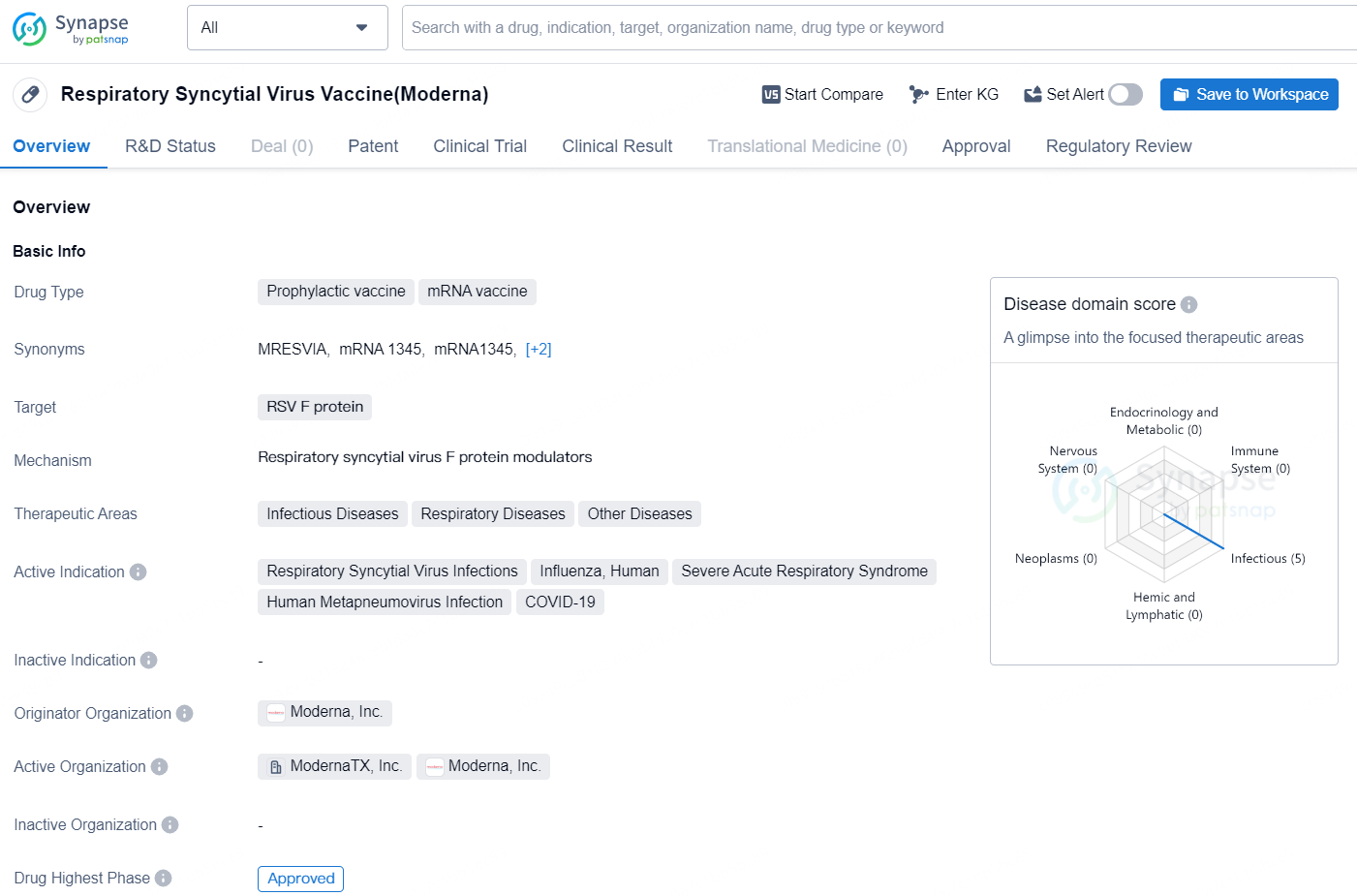
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Stéphane Bancel, Moderna’s Chief Executive Officer, expressed that the European Commission’s endorsement of mRESVIA represents a significant achievement in public health and underscores Moderna's dominance in mRNA technology. This is the first instance of an mRNA vaccine receiving approval in Europe for a disease other than COVID-19. Bancel highlighted that mRESVIA protects elderly individuals from severe RSV complications and comes uniquely pre-filled in a syringe, facilitating easier administration and potentially minimizing preparation time and errors.
Respiratory Syncytial Virus (RSV) is a highly transmissible seasonal respiratory pathogen, significantly contributing to lower respiratory tract infections and pneumonia, particularly affecting infants and older populations. In the European Union, RSV annually leads to around 160,000 hospitalizations among adults, with 92% of these cases involving individuals aged 65 and above.
The marketing authorization for mRESVIA is founded on encouraging data from the Phase 3 ConquerRSV clinical trial, a global study involving about 37,000 participants aged 60 and above across 22 countries. The primary analysis, with a median follow-up of 3.7 months, demonstrated a vaccine efficacy (VE) against RSV lower respiratory tract disease (LRTD) of 83.7% (95.88% CI 66.0%, 92.2%), with findings published in The New England Journal of Medicine. An additional analysis with a median follow-up of 8.6 months showed that mRNA-1345 continued to exhibit durable efficacy, maintaining a VE of 63.3% (95% CI: 48.7%, 73.7%) against RSV-LRTD with two or more symptoms. VE was 74.6% (95% CI, 50.7, 86.9) against RSV-LRTD with at least two symptoms, including shortness of breath, and 63.0% (95% CI, 37.3%, 78.2%) against RSV-LRTD with three or more symptoms. The study’s stringent statistical criterion, a lower bound of the 95% CI above 20%, was satisfied for both endpoints. Commonly reported adverse reactions included injection site pain, fatigue, headache, myalgia, and arthralgia.
In May 2024, the U.S. Food and Drug Administration (FDA) approved mRESVIA (mRNA-1345) to protect adults aged 60 and older from lower respiratory tract disease caused by RSV. This approval, granted under a breakthrough therapy designation, marked Moderna’s second approved mRNA product. Moderna has also submitted applications for marketing authorization of mRNA-1345 in multiple international markets.
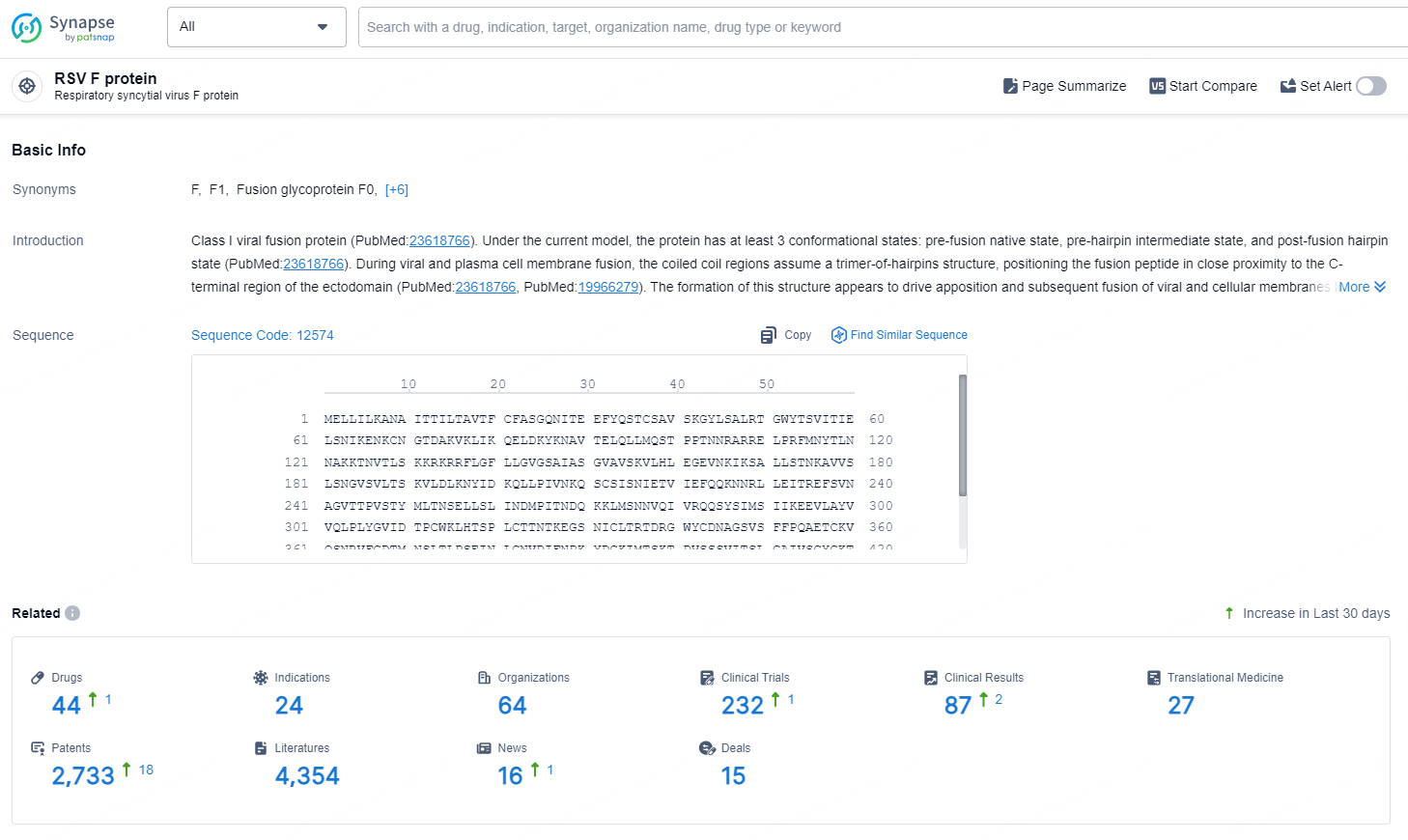
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 27, 2024, there are 44 investigational drugs for the RSV F protein targets, including 24 indications, 64 R&D institutions involved, with related clinical trials reaching 232, and as many as 2733 patents.
The Respiratory Syncytial Virus (RSV) Vaccine developed by Moderna, Inc. is a prophylactic mRNA vaccine targeting the RSV F protein. The therapeutic areas for this vaccine include infectious diseases, respiratory diseases, and other diseases. The active indications for the vaccine are Respiratory Syncytial Virus Infections, Influenza, Human, Severe Acute Respiratory Syndrome, Human Metapneumovirus Infection, and COVID-19.