Mabwell Receives CDE Nod for New Nectin-4 ADC, Set to Start Phase III Cervical Cancer Trial
Mabwell (688062.SH), a pioneering biopharmaceutical enterprise with a comprehensive industry chain, has declared that its application to the Center for Drug Evaluation (CDE) under the National Medical Products Administration (NMPA) for the study protocol titled “A Randomized, Open-label, Phase III Study to Evaluate 9MW2821 vs Investigator’s Choice of Chemotherapy in Subjects with Recurrent or Metastatic Cervical Cancer Who Progressed on or after Platinum-based Chemotherapy” has been accepted. The company will commence a Phase III clinical trial aiming to investigate the effectiveness and safety of 9MW2821 in individuals with recurrent or metastatic cervical cancer (CC) that has advanced following platinum-based chemotherapy.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
9MW2821, an innovative Nectin-4 targeting ADC formulated by Mabwell, marks the pioneering entry into clinical trials among Nectin-4-targeting ADCs created by Chinese firms. Furthermore, it is the first of its kind to progress to phase III clinical trials for treating cervical cancer on a global scale. Extensive clinical studies have been undertaken for urothelial carcinoma, cervical cancer, esophageal cancer, and breast cancer with over 400 participants enrolled. These clinical trials have revealed excellent therapeutic efficacy and safety.
Currently, therapeutic options and outcomes for patients with recurrent or metastatic cervical cancer are quite limited. In the cervical cancer subset of the phase I/II trial, the rate of Nectin-4 expression was found to be 91.87%, with a 73.98% detection rate for Nectin-4 IHC 3+. A total of 53 patients with cervical cancer received at least one dose of 9MW2821 and were deemed suitable for efficacy evaluation. All of these patients had been treated with doublet platinum-containing chemotherapy; 51% had previously been administered bevacizumab, and 58% had received immune checkpoint inhibitors. The ORR and DCR among these 53 patients were found to be 35.8% and 81.1%, respectively. Median progression-free survival (mPFS) was 3.9 months, while the median duration of response (DOR) was 7.2 months. Median overall survival (OS) has not been reached yet, with a 12-month OS rate of 74.6%. For patients exhibiting Nectin-4 IHC 3+, the ORR was 43.6%.
These findings suggest that 9MW2821 shows a beneficial therapeutic impact on cervical cancer patients. The company is presently engaged in the systematic evaluation and advancement of clinical studies focusing on first-line combination therapies.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
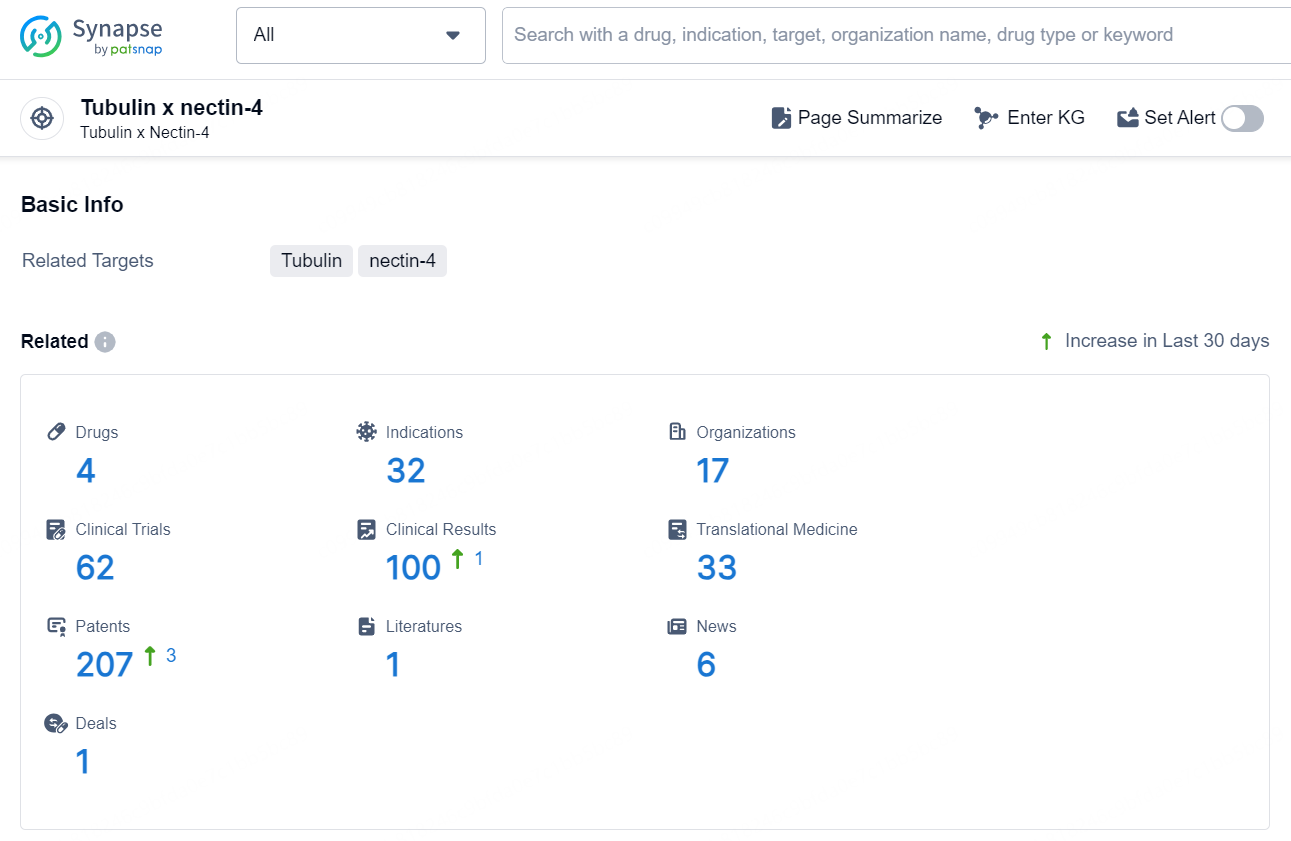
According to the data provided by the Synapse Database, As of August 27, 2024, there are 4 investigational drugs for the Tubulin x nectin-4 target, including 32 indications, 17 R&D institutions involved, with related clinical trials reaching 62, and as many as 207 patents.
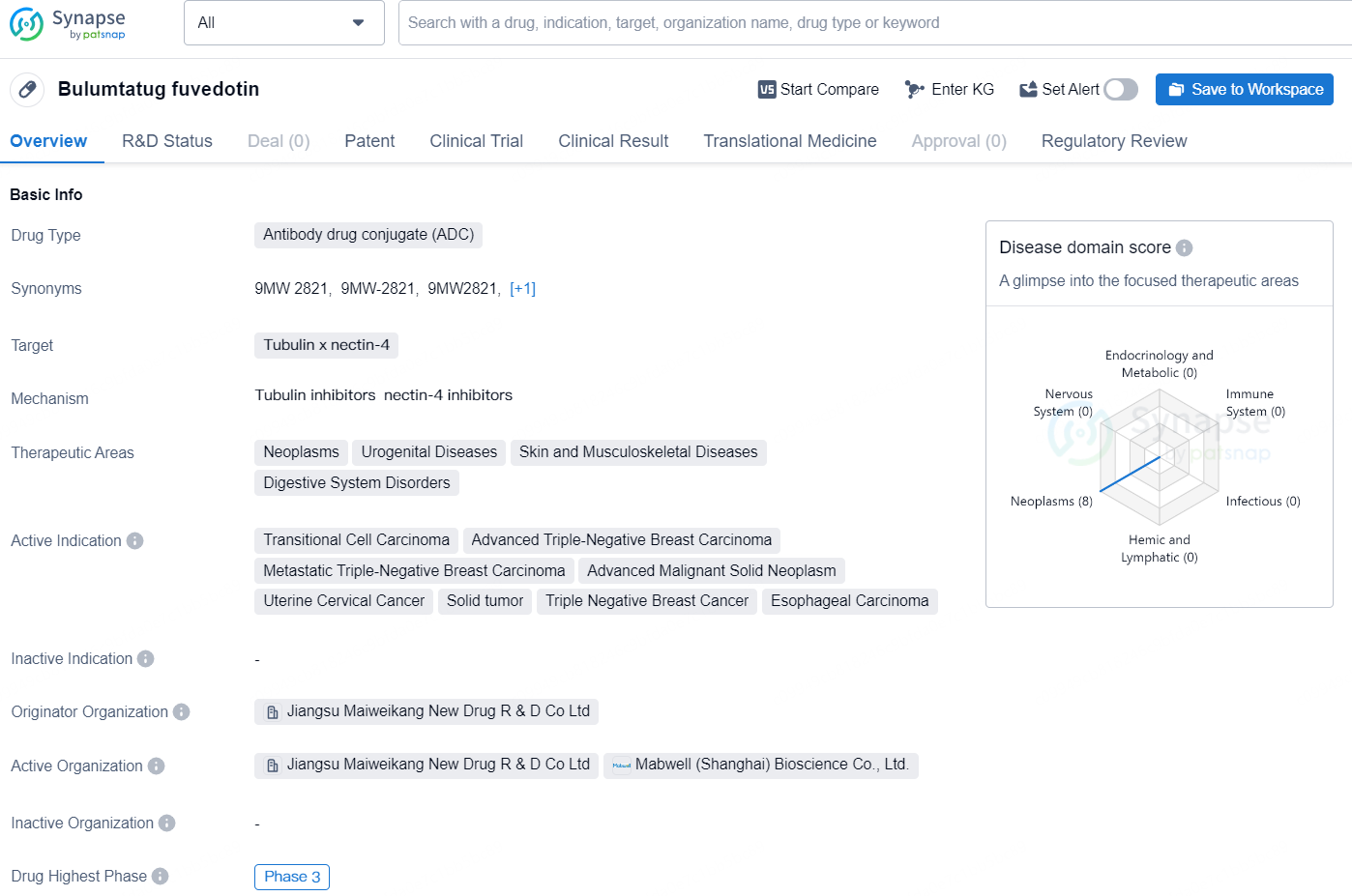
Bulumtatug fuvedotin (9MW2821) is an antibody drug conjugate (ADC) targeting tubulin and nectin-4. It is being developed for the treatment of various neoplasms and urogenital, skin, musculoskeletal, and digestive system diseases. The active indications for the drug include transitional cell carcinoma, advanced triple-negative breast carcinoma, metastatic triple-negative breast carcinoma, advanced malignant solid neoplasm, uterine cervical cancer, solid tumor, triple-negative breast cancer, and esophageal carcinoma. The drug is being developed by Jiangsu Maiweikang New Drug R & D Co Ltd and has reached Phase 3 in both the global and Chinese markets.